Professional Documents
Culture Documents
Hammett Plots Notes
Uploaded by
polypeptideCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Hammett Plots Notes
Uploaded by
polypeptideCopyright:
Available Formats
HAMMETT PLOTS substituent effects => field, resonance, inductive, polarizability and steric effects ; the first 4 are
electronic effects; together, these are stereoelectronic effects steric effects are electronic in origin since they are repulsions brought about by atoms approaching within their respective van der Waals distances, where electron clouds of the groups repel each other Hammett plots are used to picture how reaction mechanisms change as a function of electronic changes induced by substituents ; it is defined using the dissociation of benzoic acid and the ability of meta or para substituents to influence its acidity ; ortho groups are excluded to eliminate steric effects
the substituent parameter x is defined using H=0
> 0 => the substituted benzoic acid is less acidic than benzoic acid => EWG < 0 => the substituted benzoic acid is more acidic than benzoic acid => EDG usually, p > m since para substituents can influence the charge at the ipso carbon via resonance
-OH and OR groups are EW in the meta position (inductive effect) but are ED in the para position (resonance effect)
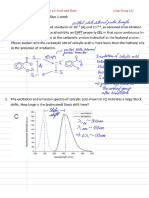
to determine if the reaction mechanism creates a positive or negative charge, the slope of the Hammett plot is defined:
describes the sensitivity of the new reaction to substituent effects relative to the influence of the substituent on the ionization of benzoic acid >1 => more sensitive to substituents than benzoic acid and negative charge is building up 0 < < 1 => less sensitive to substituents than benzoic acid but negative charge is building up ~0 => no substituent effects => no change occurs in the RDS < 0 => build up of positive charge
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- NMR Yield CalculationDocument4 pagesNMR Yield CalculationpolypeptideNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Glass Care Safe Handling PDFDocument16 pagesGlass Care Safe Handling PDFAdinda SaraswatiNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Homework 3Document13 pagesHomework 3polypeptideNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- Viral Diseases MechanismsDocument105 pagesViral Diseases MechanismspolypeptideNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Chemical Equilibrium (Final Review)Document20 pagesChemical Equilibrium (Final Review)polypeptideNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Aic I HW7Document2 pagesAic I HW7polypeptideNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Homework 3Document13 pagesHomework 3polypeptideNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Glass Care Safe Handling PDFDocument16 pagesGlass Care Safe Handling PDFAdinda SaraswatiNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Chem 14A Discussion Section: Coordination ChemistryDocument3 pagesChem 14A Discussion Section: Coordination ChemistrypolypeptideNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- Units of Measurement: Introduction To ChemistryDocument4 pagesUnits of Measurement: Introduction To ChemistrypolypeptideNo ratings yet
- Lecture of Zoonoses at NTU 2015Document159 pagesLecture of Zoonoses at NTU 2015polypeptideNo ratings yet
- Midterm RecitationDocument12 pagesMidterm RecitationpolypeptideNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Animal ConditioningDocument27 pagesAnimal ConditioningpolypeptideNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Mechanism of Microbial InfectionsDocument122 pagesMechanism of Microbial InfectionspolypeptideNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- pKa Table AnalysisDocument6 pagespKa Table AnalysisAlessandro MoreniNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Chapter 28 LCDocument5 pagesChapter 28 LCpolypeptideNo ratings yet
- Functional Groups in Organic ChemistryDocument1 pageFunctional Groups in Organic ChemistrygznmemberNo ratings yet
- General Chemistry C Homework 6 Due On 12/11 Name: ID: DepartmentDocument2 pagesGeneral Chemistry C Homework 6 Due On 12/11 Name: ID: DepartmentpolypeptideNo ratings yet
- Chapter24 Materials ChemDocument25 pagesChapter24 Materials ChempolypeptideNo ratings yet
- Chemistry GRE SampleDocument0 pagesChemistry GRE Sampleyoostan100% (2)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Protective GroupDocument26 pagesProtective Groupchhan4No ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Chapter 7 Intro To Optical InstrumentsDocument10 pagesChapter 7 Intro To Optical InstrumentspolypeptideNo ratings yet
- Est-Ce QueDocument6 pagesEst-Ce QuepolypeptideNo ratings yet
- Chapter1s StatMechDocument22 pagesChapter1s StatMechpolypeptideNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- C H ActivationDocument55 pagesC H Activationpolypeptide100% (1)
- Test Taking StrategiesDocument193 pagesTest Taking StrategiesAmy Kennedy100% (1)
- FLUXIONALITYDocument7 pagesFLUXIONALITYpolypeptideNo ratings yet
- Alkyne Metathesis & Enyne MetathesisDocument3 pagesAlkyne Metathesis & Enyne MetathesispolypeptideNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- E26 QuestionsDocument5 pagesE26 QuestionspolypeptideNo ratings yet
- Literature Report 2Document12 pagesLiterature Report 2polypeptideNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)