Professional Documents
Culture Documents
Isomerism in Alkanes, Cycloalkanes, and Alkenes Using Molecular Models
Uploaded by
albertvdatu278Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Isomerism in Alkanes, Cycloalkanes, and Alkenes Using Molecular Models
Uploaded by
albertvdatu278Copyright:
Available Formats
EXPERIMENT
Isomerism in Alkanes, Cycloalkanes, and Alkenes using Molecular Models
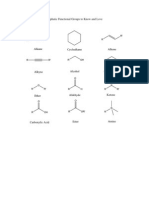
Materials Needed - Molecular model kit PRE-LABORATORY QUESTIONS 1. Draw structural formulas of (a) n-hexane (b) 1-hexene (c) cyclohexane (d) cyclohexene.
2. Which if any of the above are isomers of each other? Explain your answer fully.
Constitutional Isomerism 1. Positional Isomerism of Haloalkanes. The models you will prepare in this section require four different items from the kit: tetrahedral carbons, hydrogens, chlorines, and sticks for single bonds. A. Methanes. Make a model for each of the following structures:
Are all four of the above superimposable on each other? ______ Do the four formulas represent different molecules? ______ Now make a model for each of the following structural formulas:
Are all four of the models superimposable on each other? ______ Do the four formulas represent different molecules? ______ Chloroform is CHCl3. How many different molecules are possible for CHCl3? ______ B. Ethanes. Prepare models of each of the molecules shown below..
Are the two models superimposable? Remember that rotation of the C-C bond easily occurs. ______ Are the two molecules isomers or the same compound? ______ What is the IUPAC name of this compound? ________________________________
Now rearrange one of the models to form the molecule shown below.
Is the new model superimposable on the one you did nothing to? __________ What is the name of the compound represented by the new model? __________________________ You now have models of two molecules that are isomers of each other: they are different compounds (with different names) that have the same molecular formula (C2H4Cl2). Furthermore, because the only difference in their structures is the position of a substituent (the chlorine) these isomers are considered to be positional isomers of each other. Post-lab assignment: Using a chemical handbook or internet reference source to fill out the following table illustrating the different physical properties of these two compounds. Compound Name literature boiling point (C) literature melting point (C)
Reference used ____________________________________________________________________ C. Propanes. Prepare a model of propane, C3H8. Can the three carbons and eight hydrogens be arranged to form more than one different molecular structure that are not merely different conformations of the same compound? ____________ Now replace any one hydrogen attached to an end carbon on the molecule with a chlorine atom. Draw the structural formula for the molecule in the space below, and label it with its proper name.
Prepare another propane molecule and then replace any one hydrogen attached to the middle carbon with a chlorine atom. Draw its structural formula, and label it with its proper name.
Are the two chloropropane molecules that you have just prepared superimposable? __________ Are they isomers? _________ If so, what type of isomerism do they exhibit? ________________ D. Butanes. Replace the chlorine atoms in your two chloropropane molecules with methyl (CH3) groups. Draw structural formulas for the two positional isomers of butane you have just prepared, and give IUPAC names for them.. Note that one of the isomers contains an unbranched carbon chain while the other has a branch in the middle. The unbranched isomer is commonly called n-butane while the branched molecule is called isobutane . For each of the structures below, indicate whether it is n-butane, isobutane, or neither.
_________________
______________
______________
_________________
_________________
________________
Post lab assignment: Give structures for n-pentane, and its two isomers. Note that one of the isomers contains a quaternary (4) carbon atom. Name all three isomers.
Name: 1. Cis-Trans Isomerism of Alkenes
Name:
Name:
(1) Ethene. Prepare two models of ethene, C2H4. Is ethene an isomer of ethane? ____________ Can you rotate the molecule around the double bond? ____________ (2) Propene. Now substitute a methyl group for one of the hydrogen atoms in each of the ethene models to form a model of propene. Are the two models superimposable? ____________ Does it matter which hydrogen in ethene you replace with the methyl group? (Is there any way to make a propene model that is not superimposable on the first one?) ____________ What is the C-C-C bond angle approximately? ____________ Give a structure for propene that shows the C-C-C bond angle.
(3) Butenes. There are four butene isomers, C4H8. You can come up with a model of each by in turn replacing different hydrogens on propene with a methyl group. Prepare the four isomers of butene, give their structures (show C-C-C bond angles realistically please), and name them by IUPAC rules. Structural formula 1. Name
2.
3.
4.
Notice that there are two isomers that have a double bond between the second and third carbon atoms of a chain. The compound with both methyl groups on the same side of the double bond is cis-2-butene (cis = on this side), and the one with the methyl groups on opposite sides of the double bond is trans-2-butene (trans = across). These are different compounds due to the fact that rotation around carbon-carbon double bonds is difficult and does not ordinarily occur, a fact accurately depicted by your models. Isomers of this type are called cis/trans isomers. (Remember, though that the structures shown below, which would be alkanes analogous to cis- and trans-2- butene, are not isomers but are the same molecule in different conformations due to the fact that rotation can occur around carbon-carbon single bonds.)
Cycloalkanes (1) Cyclohexane. Make a ring of six carbon atoms using single bonds. Fill in the remaining positions with hydrogen. This molecule is cyclohexane. Cyclohexane can exist in the two non-planar forms shown below.
Boat form
Chair form
Change your model back and forth between the chair and boat forms. This process occurs very rapidly at room temperature. What is the relationship between the chair and the boat form of cyclohexane? A. stereoisomers B. structural isomers C. different conformations of the same molecule
The chair conformation is much more stable than the boat. Thus, cyclohexane mainly exists in the chair conformation. Answer the following questions by looking at your model of the chair conformation. What are the bond angles around the carbon atoms? _________ What is the preferred bond angle for these carbon atoms as predicted by VSEPR theory? ________ Is cyclohexane a stable molecule? __________ (2) Cyclopropane. Make a ring of three carbon atoms using single bonds. Fill in the remaining positions with hydrogen. This molecule is cyclopropane. Draw the structure below.
What are the C-C-C bond angles in cyclopropane? ___________ What is the preferred bond angle for these carbon atoms as predicted by VSEPR theory? ________ Is cyclopropane a stable molecule? __________
You might also like
- Understanding IsomerismDocument40 pagesUnderstanding IsomerismHafizszulfeyzul Feyzul100% (1)
- CAPE Unit 2 Chemistry NotesDocument207 pagesCAPE Unit 2 Chemistry NotesAshley Cunningham100% (2)
- General Chemistry 1 Qt. 2 Week 5Document31 pagesGeneral Chemistry 1 Qt. 2 Week 5Nina Reca OmisolNo ratings yet
- AppointmentDocument7 pagesAppointmentalbertvdatu278No ratings yet
- BL3B User Manual PDFDocument142 pagesBL3B User Manual PDFRandy VanegasNo ratings yet
- Topic 11 - Introduction To Organic ChemistryDocument102 pagesTopic 11 - Introduction To Organic ChemistryMohamad AzzmerNo ratings yet
- Deped Order No 42 Series of 2016 Policy Guidelines On Lesson Preparation For The K To 12 Basic Education ProgramDocument62 pagesDeped Order No 42 Series of 2016 Policy Guidelines On Lesson Preparation For The K To 12 Basic Education Programapi-24731894792% (26)
- CIP PresentationDocument17 pagesCIP Presentationalbertvdatu278100% (1)
- A. What Is Balanced/objective Review or Criticism?Document11 pagesA. What Is Balanced/objective Review or Criticism?Risha Ann CortesNo ratings yet
- IB Topic 10: Organic Chemistry Practice QuestionsDocument36 pagesIB Topic 10: Organic Chemistry Practice Questionshunarsandhu50% (2)
- Molecular Models5pDocument5 pagesMolecular Models5pAubrey Love LabardaNo ratings yet
- Molecmod LabDocument7 pagesMolecmod Labraym6270No ratings yet
- Practical 3Document8 pagesPractical 3KINISHAA A/P TAMIL SELVEN / UPMNo ratings yet
- Practical 4Document9 pagesPractical 4KINISHAA A/P TAMIL SELVEN / UPMNo ratings yet
- Organic Chemistry Student Booklet 2Document15 pagesOrganic Chemistry Student Booklet 2Mirjeta ZymeriNo ratings yet
- Chem Exam 4Document30 pagesChem Exam 4TomNo ratings yet
- Hydrocarbons Structure & Nomenclature QuestionsDocument8 pagesHydrocarbons Structure & Nomenclature Questionsleonard emanuelNo ratings yet
- E4 StereoisomersDocument6 pagesE4 StereoisomersShaun Martel BantuganNo ratings yet
- Fuel MoleculesDocument3 pagesFuel MoleculesPhillip CookNo ratings yet
- Hydrocarbons GuideDocument24 pagesHydrocarbons GuideTomNo ratings yet
- Group Logo Chem 23.1 Inorganic Analytical Chemistry Laboratory Isomerism and Stereochemistry Worksheet AnswersDocument4 pagesGroup Logo Chem 23.1 Inorganic Analytical Chemistry Laboratory Isomerism and Stereochemistry Worksheet AnswersJade AsparinNo ratings yet
- Student Study Guide and Solution Manual For Organic Chemistry 4e by David Klein 9 76Document68 pagesStudent Study Guide and Solution Manual For Organic Chemistry 4e by David Klein 9 76MA. FRANCESCA DUCOTNo ratings yet
- Organic Isomerism ExplainedDocument5 pagesOrganic Isomerism ExplainedslixsterNo ratings yet
- Molecular Modeling: Isomers, Conformers and StereoisomersDocument17 pagesMolecular Modeling: Isomers, Conformers and StereoisomersGoh Chun KitNo ratings yet
- Assessments and Rubrics For Unit 2Document13 pagesAssessments and Rubrics For Unit 2api-302258576No ratings yet
- Molecular Structures Water and PH LabDocument13 pagesMolecular Structures Water and PH Labapi-249772989No ratings yet
- Intro To Organic POGILDocument6 pagesIntro To Organic POGILzfmwj2db47No ratings yet
- Modul 1 Intro To CDocument32 pagesModul 1 Intro To CUng Hie HuongNo ratings yet
- Stereochemistry Lab: Isomers & Molecular ModelsDocument19 pagesStereochemistry Lab: Isomers & Molecular Modelssophia del rosarioNo ratings yet
- Chapter 21 Student NotesDocument10 pagesChapter 21 Student Notesapi-307565882No ratings yet
- Carbs: Structures & IsomersDocument20 pagesCarbs: Structures & IsomersSurajit BhattacharjeeNo ratings yet
- Chapter 1. Structure and BondingDocument6 pagesChapter 1. Structure and BondinganeettaNo ratings yet
- Chemistry Final Exam Review 2012Document29 pagesChemistry Final Exam Review 2012elenaNo ratings yet
- Topic 10 Notes (1)Document34 pagesTopic 10 Notes (1)Catherine Lam PoklepovicNo ratings yet
- Science Bowl Organic Chemistry NotesDocument44 pagesScience Bowl Organic Chemistry Notestaosat11No ratings yet
- VSEPRActivity 06Document6 pagesVSEPRActivity 06ᜆᜑᜒᜇᜒᜐ ᜉᜀᜎᜃᜒᜓNo ratings yet
- (Trans) Chem 33 1 NomenclatureDocument63 pages(Trans) Chem 33 1 NomenclatureALongNo ratings yet
- Hydrocarbons: Alkanes, Alkenes, Aromatic CompoundsDocument5 pagesHydrocarbons: Alkanes, Alkenes, Aromatic CompoundsAhmad asaNo ratings yet
- Unit 10R - CarbohydratesDocument20 pagesUnit 10R - CarbohydratesGovind ManglaniNo ratings yet
- 11th Class Chemistry Chapter 12 Study MaterialDocument49 pages11th Class Chemistry Chapter 12 Study MaterialAyushi ShahNo ratings yet
- Neet Chemistry Notes-1Document37 pagesNeet Chemistry Notes-1Abhishek KumarNo ratings yet
- Stereo ChemistryDocument17 pagesStereo ChemistryDeepak PradhanNo ratings yet
- Module 1. The Chemistry of Carbon Compounds TOPIC: Structure and FormulaeDocument38 pagesModule 1. The Chemistry of Carbon Compounds TOPIC: Structure and FormulaeOrlanda EllisNo ratings yet
- S1 Gchem Practical Shapes of MoleculesDocument8 pagesS1 Gchem Practical Shapes of MoleculesNorhadi MohamadNo ratings yet
- Sch4uc Unit 2 Lesson 06Document20 pagesSch4uc Unit 2 Lesson 06Luis David Lazo CondoriNo ratings yet
- 11.0 Introduction To Organic ChemDocument13 pages11.0 Introduction To Organic Chempearl ikebuakuNo ratings yet
- Long Exam 1Document8 pagesLong Exam 1Allan DNo ratings yet
- 4.B Isomerism (As)Document13 pages4.B Isomerism (As)ytshortsfromopus65No ratings yet
- ChemistryDocument166 pagesChemistryjakesidhuNo ratings yet
- Chapter 3 Alkanes, Cycloalkanes, Alkenes, Alkynes Updated 20222Document167 pagesChapter 3 Alkanes, Cycloalkanes, Alkenes, Alkynes Updated 20222Yusra IqbalNo ratings yet
- Leaning Packet 6 Engg Chem 1Document42 pagesLeaning Packet 6 Engg Chem 1Ritchel Conde BoholNo ratings yet
- Chem204 Lecture 1 EnglishDocument32 pagesChem204 Lecture 1 EnglishДууяа Б.No ratings yet
- UntitledDocument46 pagesUntitled양우경No ratings yet
- Chemistry Revision Worksheet GR 8&9Document10 pagesChemistry Revision Worksheet GR 8&9kashif mohammedNo ratings yet
- Report 1 Learning Software - Benjamin CruzDocument12 pagesReport 1 Learning Software - Benjamin Cruzapi-674986426No ratings yet
- Organic Chemistry NotesDocument59 pagesOrganic Chemistry NotesAakif RazaNo ratings yet
- CH - 4 Carbon and Its CompoundsDocument9 pagesCH - 4 Carbon and Its Compoundsbarnalisharmah586No ratings yet
- 4.a Intro Organic ChemDocument16 pages4.a Intro Organic Chemytshortsfromopus65No ratings yet
- Chapter 14 - An Introduction To Organic ChemistryDocument29 pagesChapter 14 - An Introduction To Organic ChemistryNabindra RuwaliNo ratings yet
- Aromatic CompoundsDocument6 pagesAromatic CompoundsSANIA FAJAR KHANNo ratings yet
- Apch11 EstersDocument2 pagesApch11 Estersloly62006No ratings yet
- C9e Answers Active Reading 04Document4 pagesC9e Answers Active Reading 04Jordan Camina75% (8)
- Basic Organic ChemistryDocument62 pagesBasic Organic ChemistrySempija AaronNo ratings yet
- Computational Pharmaceutics: Application of Molecular Modeling in Drug DeliveryFrom EverandComputational Pharmaceutics: Application of Molecular Modeling in Drug DeliveryDefang OuyangNo ratings yet
- Pampanga Certificate of Non-Availability of Stocks: Product Code Product Description UOM PriceDocument6 pagesPampanga Certificate of Non-Availability of Stocks: Product Code Product Description UOM Pricealbertvdatu278No ratings yet
- Sample POWDocument31 pagesSample POWJitherDoromalDelaCruzNo ratings yet
- List of Common Supplies April 19Document13 pagesList of Common Supplies April 19albertvdatu278No ratings yet
- Quick Count Enrollment 2021 2022Document11 pagesQuick Count Enrollment 2021 2022albertvdatu278No ratings yet
- Supervisory PlanDocument2 pagesSupervisory Planalbertvdatu278No ratings yet
- Assessment ToolsDocument2 pagesAssessment Toolsalbertvdatu278No ratings yet
- CIP TitlesDocument1 pageCIP Titlesalbertvdatu278No ratings yet
- CI Program Justino Sevilla HSDocument1 pageCI Program Justino Sevilla HSalbertvdatu278No ratings yet
- Waste Material ReportDocument1 pageWaste Material Reportalbertvdatu278No ratings yet
- Courses To OfferDocument1 pageCourses To Offeralbertvdatu278No ratings yet
- Revolutionary Engineer & GeneralDocument3 pagesRevolutionary Engineer & Generalalbertvdatu278No ratings yet
- Whole Brain TeachingDocument1 pageWhole Brain Teachingalbertvdatu278No ratings yet
- Macromolecules Worksheets Biology ClassDocument8 pagesMacromolecules Worksheets Biology Classalbertvdatu278No ratings yet
- Lesson Plan - Gas Laws - HighlightedDocument5 pagesLesson Plan - Gas Laws - Highlightedalbertvdatu278No ratings yet
- Room Examiner's WorkflowDocument19 pagesRoom Examiner's Workflowalbertvdatu278No ratings yet
- 3 Year Plan MatrixDocument2 pages3 Year Plan Matrixalbertvdatu278No ratings yet
- Acids Bases Practice Problems MCDocument24 pagesAcids Bases Practice Problems MCalbertvdatu278No ratings yet
- Waste Management Collaboration RubricDocument2 pagesWaste Management Collaboration Rubricalbertvdatu278No ratings yet
- 3 Year Plan MatrixDocument2 pages3 Year Plan Matrixalbertvdatu278No ratings yet
- 1 ChoDocument38 pages1 ChoFauzan FasnidNo ratings yet
- Action Research WorkshopDocument3 pagesAction Research Workshopalbertvdatu278No ratings yet
- TSGE - TLGE - TTGE - Reduce Moment High Performance CouplingDocument6 pagesTSGE - TLGE - TTGE - Reduce Moment High Performance CouplingazayfathirNo ratings yet
- COP2251 Syllabus - Ellis 0525Document9 pagesCOP2251 Syllabus - Ellis 0525Satish PrajapatiNo ratings yet
- AIIMS Mental Health Nursing Exam ReviewDocument28 pagesAIIMS Mental Health Nursing Exam ReviewImraan KhanNo ratings yet
- Research of William Wells at HarvardDocument10 pagesResearch of William Wells at HarvardARGHA MANNANo ratings yet
- Reaction CalorimetryDocument7 pagesReaction CalorimetrySankar Adhikari100% (1)
- Manuais - 727312 - manuais-Raios-X AXR - 77000001249Document72 pagesManuais - 727312 - manuais-Raios-X AXR - 77000001249Hosam Ahmed HashimNo ratings yet
- 1 Univalent Functions The Elementary Theory 2018Document12 pages1 Univalent Functions The Elementary Theory 2018smpopadeNo ratings yet
- Destroyed Inventory Deduction ProceduresDocument7 pagesDestroyed Inventory Deduction ProceduresCliff DaquioagNo ratings yet
- TESTIS PHYSIOLOGY Spermatogenic Cell Syncytium Makela and Toppari 2018Document10 pagesTESTIS PHYSIOLOGY Spermatogenic Cell Syncytium Makela and Toppari 2018LudimilaNo ratings yet
- Module 3 Paired and Two Sample T TestDocument18 pagesModule 3 Paired and Two Sample T TestLastica, Geralyn Mae F.No ratings yet
- 2113T Feasibility Study TempateDocument27 pages2113T Feasibility Study TempateRA MagallanesNo ratings yet
- Offshore Wind Turbine 6mw Robust Simple EfficientDocument4 pagesOffshore Wind Turbine 6mw Robust Simple EfficientCristian Jhair PerezNo ratings yet
- Course Handbook MSC Marketing Sept2022Document58 pagesCourse Handbook MSC Marketing Sept2022Tauseef JamalNo ratings yet
- Unitary Small Air-Conditioners and Air-Source Heat Pumps (Includes Mixed-Match Coils) (RATED BELOW 65,000 BTU/H) Certification ProgramDocument65 pagesUnitary Small Air-Conditioners and Air-Source Heat Pumps (Includes Mixed-Match Coils) (RATED BELOW 65,000 BTU/H) Certification ProgramAmer GaladNo ratings yet
- Team Dynamics and Behaviors for Global ExpansionDocument15 pagesTeam Dynamics and Behaviors for Global ExpansionNguyênNo ratings yet
- FRABA - Absolute - Encoder / PLC - 1 (CPU 314C-2 PN/DP) / Program BlocksDocument3 pagesFRABA - Absolute - Encoder / PLC - 1 (CPU 314C-2 PN/DP) / Program BlocksAhmed YacoubNo ratings yet
- Case Study - Help DocumentDocument2 pagesCase Study - Help DocumentRahNo ratings yet
- CONNECTIFYDocument3 pagesCONNECTIFYAbhishek KulshresthaNo ratings yet
- After EffectsDocument56 pagesAfter EffectsRodrigo ArgentoNo ratings yet
- O - 6 Series Mill Operation Manual-ENDocument119 pagesO - 6 Series Mill Operation Manual-ENLeonardo OlivaresNo ratings yet
- Costing - Type Wise Practical Mcq-Executive-RevisionDocument71 pagesCosting - Type Wise Practical Mcq-Executive-RevisionShruthi ParameshwaranNo ratings yet
- Trabajo de Investigación FormativaDocument75 pagesTrabajo de Investigación Formativalucio RNo ratings yet
- Data Validation and Verification - BBC BitsizeDocument56 pagesData Validation and Verification - BBC BitsizeluciferothegoatNo ratings yet
- Conservation of Kuttichira SettlementDocument145 pagesConservation of Kuttichira SettlementSumayya Kareem100% (1)
- Siemens MS 42.0 Engine Control System GuideDocument56 pagesSiemens MS 42.0 Engine Control System GuideIbnu NugroNo ratings yet
- 1 s2.0 S0959652619316804 MainDocument11 pages1 s2.0 S0959652619316804 MainEmma RouyreNo ratings yet
- Evolution BrochureDocument4 pagesEvolution Brochurelucas28031978No ratings yet
- An RNA Vaccine Drives Expansion and Efficacy of claudin-CAR-T Cells Against Solid TumorsDocument9 pagesAn RNA Vaccine Drives Expansion and Efficacy of claudin-CAR-T Cells Against Solid TumorsYusuf DemirNo ratings yet