Professional Documents
Culture Documents
Temp Vs Heat - Student Handouts
Uploaded by
api-233355384Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Temp Vs Heat - Student Handouts
Uploaded by
api-233355384Copyright:
Available Formats
Is There a Difference Between Temperature and Heat?
Part 1 Do Now With your partner, answer the following: 1) Which object contains the most amount of heat, a pot of boiling water, or a gigantic iceberg? Why?
2) Youre at the beach. The temperature of the air is 101F. The sand burns your feet (ouch!) and the ocean makes you shiver. Why? 3) Why does rubbing alcohol feel colder than room temperature when put on your skin? (dont believe me? Ill show you!) 4) True/False the temperature of something depends on its size 5) True/False - Heat flows from an area of low temperature to an area of high temperature Draw a picture to show how temperature moves (Hint: what happens when you pour boiling water into ice water?)
5) True/False - When heat transfer happens, the object with the higher temperature experiences a decrease in temperature. Why? 6) True/False Water and Vegetable Oil will require the same amount of heat to raise the temperature 7) Draw a picture of what water molecules look like as a solid, liquid, and gas:
Solid
Liquid
Gas
Part 2 Lab Challenge
Using the materials provided, the data tables below, and the hints to get you started, determine if there is a difference between temperature and heat, and make a hypothesis for what the difference is. Materials 25 mL Water 25 mL Vegetable Oil 2 Candles/group Tongs (to hold candle) 100 mL Beaker
Wire stand Scale Calculator Timer/stopwatch Safety goggles
Keep your face away from heating liquids! Hold candles with tongs! WEAR YOUR GOGGLES
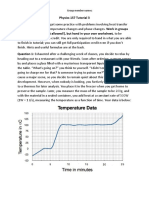
A few hints to get you started: Use one candle to raise the temperature of each liquid. Take a temperature reading every 30 seconds for 4 minutes and record in Data Table 2. Fill in the data tables as you go.
Data Table 1
Water
Vegetable Oil
Mass of Beaker (g) Mass of Liquid (g) in beaker Mass of Liquid only (g) Initial temperature (C) Initial mass of candle (g) Final temperature (C) Final mass of candle (g)
Data Table 2
0 sec
30 sec
60 sec
90 sec
120 sec
150 sec
180 sec
210 sec
240 sec
Water C Oil C
Initial hypothesis: The difference between temperature and heat is
Raise your hand to show Meghan your hypothesis then shell give you what you need to move on!
Part 3 Calculating Heat
Well, the truth is we cant measure heat using a thermometer like we can for temperature, BUTit can be calculated. The formula for determining the amount of heat is q=mC T Thats the fancy way of saying that: Heat = (mass) x (specific heat) x (the change in temperature) Where, q= heat m = mass of liquid (g) C= specific heat constant (see below) T= (final temperature) (initial temperature) The specific heat (C) is a constant for a given substance its the amount of heat needed to raise the temperature of one gram of that substance by 1 degree Celsius.
Specific Heat of Water = 1 calorie/gram C Specific Heat of Vegetable Oil = 0.46 calories/gram C
* A calorie is a unit of energy
Using this formula and the results from your experiments, determine how much heat it took to raise the temperature of each liquid in 4 minutes. Water Amount of Heat q=mCT Vegetable Oil
If youre having trouble with the calculations, see Meghan, Eric, or one of the Antioch students for a Hint Card
Is your hypothesis {the difference between temperature and heat} the same after doing the calculations? If not, write a new hypothesis and explain why you changed your mind.
When you are done, quickly clean up your lab station and grab the MINDBENDERS from the front to work on while the rest of the class finishes up
You might also like
- Pogil Heat and CalorimetryDocument4 pagesPogil Heat and Calorimetryapi-341706426No ratings yet
- Temperature and Thermal Equilibrium LAB 1Document10 pagesTemperature and Thermal Equilibrium LAB 1Roselyn BenavidezNo ratings yet
- Experiment 11 Specific Heat and Heat of Fusion: PreparationDocument5 pagesExperiment 11 Specific Heat and Heat of Fusion: PreparationMae Joy PalmaNo ratings yet
- Calorimetry methods for measuring heatDocument6 pagesCalorimetry methods for measuring heatJAMAICA MARIE DURANNo ratings yet
- Vernier-Mixing Warm ColdDocument4 pagesVernier-Mixing Warm ColdOeng BunhakNo ratings yet
- Heat of Fusion of IceDocument2 pagesHeat of Fusion of IceReyna Federo100% (1)
- 1045 Exp8 CalorimetryDocument15 pages1045 Exp8 CalorimetryLei LopezNo ratings yet
- Math 5 Quarter 4 Week 6Document62 pagesMath 5 Quarter 4 Week 6Imee Moran Long100% (1)
- Reading TemperaturesDocument35 pagesReading TemperaturesLeonida Villalon100% (1)
- 6 Heat of Combust and Phase W PictureDocument4 pages6 Heat of Combust and Phase W Pictureaeliaeli29No ratings yet
- Dystan Medical Supply Company - Cold Packs and Hot Packs Lab ReportDocument10 pagesDystan Medical Supply Company - Cold Packs and Hot Packs Lab ReportVivek Patel80% (5)
- Home Experiment For Measuring The Latent Heat of IceDocument2 pagesHome Experiment For Measuring The Latent Heat of IcechlronaldNo ratings yet
- Avin Maroozi - Lesson 5Document4 pagesAvin Maroozi - Lesson 5Avin MarooziNo ratings yet
- Module 13 Heat and Temperature ConceptsDocument37 pagesModule 13 Heat and Temperature ConceptsMelvin Cabonegro100% (2)
- Exp 4 Heat of Fusion-Melting IceDocument7 pagesExp 4 Heat of Fusion-Melting IceEngelbert AddonganNo ratings yet
- Flower Duet in G Voices OnlyDocument80 pagesFlower Duet in G Voices Onlym1d0r1No ratings yet
- Heat and Temperature Activity 1 Explaining Hotness and ColdnessDocument21 pagesHeat and Temperature Activity 1 Explaining Hotness and ColdnessChristopher M. CasuguidNo ratings yet
- Experiment 6 CalorimetryDocument4 pagesExperiment 6 CalorimetryJoshua EgallaNo ratings yet
- GC2 Thermodynamics and CalorimetryDocument44 pagesGC2 Thermodynamics and CalorimetryAkisha FijoNo ratings yet
- CaliometryDocument14 pagesCaliometryshivanshkumarsahu07No ratings yet
- Determine Specific Heats of LiquidsDocument9 pagesDetermine Specific Heats of LiquidsSai Swetha KVNo ratings yet
- Latent Heat of FusionDocument3 pagesLatent Heat of FusionJamiel Catapang100% (1)
- Temperature and Thermal Energy ExplainedDocument8 pagesTemperature and Thermal Energy ExplainedJessa Sumaylo CalambaNo ratings yet
- Heat of Fusion of WaterDocument6 pagesHeat of Fusion of WaterAishaNo ratings yet
- Unit 02 LAB Heat of Fusion of IceDocument4 pagesUnit 02 LAB Heat of Fusion of IceJesiann SmithNo ratings yet
- Heat and Calories Simulation PDFDocument3 pagesHeat and Calories Simulation PDFvinod kumarNo ratings yet
- LAB4 The Conservation of Thermal EnergyDocument10 pagesLAB4 The Conservation of Thermal EnergyMark Adrian TagabanNo ratings yet
- Name: Group Member Names: Student NumberDocument6 pagesName: Group Member Names: Student NumberSherin HamidNo ratings yet
- Measuring Temperature MathDocument8 pagesMeasuring Temperature MathGenalyn Tambiao AchanzarNo ratings yet
- Heat of Neutralization LabDocument2 pagesHeat of Neutralization Labmunira9450% (2)
- Applying The Concept of Balanced Diet: NutritionDocument13 pagesApplying The Concept of Balanced Diet: NutritionNurNo ratings yet
- Corrocher Patric Trending DataDocument4 pagesCorrocher Patric Trending DataJackie McCarthyNo ratings yet
- Specific HeatDocument2 pagesSpecific HeatJorge Rodríguez SedanoNo ratings yet
- David Fabián Guerrero Díaz A00571065: Calculating Mas in Base of Temperature ChangeDocument2 pagesDavid Fabián Guerrero Díaz A00571065: Calculating Mas in Base of Temperature ChangePacoNo ratings yet
- Calorie Lab: Measure Energy in FoodDocument2 pagesCalorie Lab: Measure Energy in FoodAllyssa De AmaNo ratings yet
- Lab Heat EnergyDocument8 pagesLab Heat EnergyFaruk IbrahimovicNo ratings yet
- 19c Latent Heat of FusionDocument3 pages19c Latent Heat of FusionAndrae Tennant100% (1)
- PHYS 1401 General Physics I Experiment 13 Latent Heat of FusionDocument4 pagesPHYS 1401 General Physics I Experiment 13 Latent Heat of FusionAlicia HaughtonNo ratings yet
- Calorimetry Lab1Document6 pagesCalorimetry Lab1api-273644689No ratings yet
- Lit Cit Added Table 5Document13 pagesLit Cit Added Table 5Rey ann EspinosaNo ratings yet
- Explaining Hotness or ColdnessDocument2 pagesExplaining Hotness or Coldnessliagiba_abbyNo ratings yet
- Physics 2 Lab Experiment-3Document8 pagesPhysics 2 Lab Experiment-3sm shamsuddinNo ratings yet
- Physics4 01measuringtemperatureDocument4 pagesPhysics4 01measuringtemperatureapi-238185553No ratings yet
- CombustionDocument4 pagesCombustionapi-250308385No ratings yet
- Arvin Enricosec 5 ThermodynamicsDocument11 pagesArvin Enricosec 5 ThermodynamicsArvin CoirneNo ratings yet
- Thermal Equilibrium ExperimentDocument3 pagesThermal Equilibrium ExperimentFish Bone25% (8)
- 3Document7 pages3chikeruNo ratings yet
- Batang GuroDocument2 pagesBatang GuroMerida BravoNo ratings yet
- Guía TermodinámicaDocument17 pagesGuía TermodinámicamiayalacNo ratings yet
- 41 Heat-TemperatureDocument7 pages41 Heat-TemperatureChess ManNo ratings yet
- Chemisrty Power Point 1 PNC SECOND TOPICDocument24 pagesChemisrty Power Point 1 PNC SECOND TOPICMa. Sophia D GelveroNo ratings yet
- Heat of Combustion of EthanolDocument5 pagesHeat of Combustion of EthanolMaynard CortezNo ratings yet
- Calorimetry Coffee Cup ExperimentDocument8 pagesCalorimetry Coffee Cup ExperimentDana Georgiana CrivoiNo ratings yet
- CalorimetryDocument10 pagesCalorimetryDaizLee Ahmad0% (1)
- Heat of Fusion and IceDocument3 pagesHeat of Fusion and Iceapi-702229801No ratings yet
- Lab 1 - Heat of Neutralization (Che 142) PDFDocument7 pagesLab 1 - Heat of Neutralization (Che 142) PDFSyafiyatulMunawarahNo ratings yet
- Pepito, Alexis R. - Assignment 2.0-Heat and TemperatureDocument5 pagesPepito, Alexis R. - Assignment 2.0-Heat and TemperaturePEPITO, ALEXIS R.SCINo ratings yet
- Experimental Write Up TemplateDocument4 pagesExperimental Write Up TemplateJason DengNo ratings yet
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterRating: 5 out of 5 stars5/5 (1)
- Geneticsoftastepacket 1314Document9 pagesGeneticsoftastepacket 1314api-233355384No ratings yet
- Waterqualityindex-Studentpacket 1Document5 pagesWaterqualityindex-Studentpacket 1api-233355384No ratings yet
- Summit Environmentallawandlegislation1314Document2 pagesSummit Environmentallawandlegislation1314api-233355384No ratings yet
- Genetics Curriculum Meghan 2013Document67 pagesGenetics Curriculum Meghan 2013api-233355384No ratings yet
- Faculty Faculty of Degree Engineering - 083: High Voltage Engineering (2160904) Multiple Choice QuestionsDocument33 pagesFaculty Faculty of Degree Engineering - 083: High Voltage Engineering (2160904) Multiple Choice QuestionsPiyush BadjateNo ratings yet
- NCERT Solutions For Class 9th Science - Chapter 9 Force and Laws of MotionDocument18 pagesNCERT Solutions For Class 9th Science - Chapter 9 Force and Laws of MotionTambramNo ratings yet
- Joule Thomson EffectDocument2 pagesJoule Thomson EffectElliott100% (1)
- Experiment Rotation: ObjectivesDocument10 pagesExperiment Rotation: ObjectivesMerryNo ratings yet
- Module 10 Electric CurrentDocument13 pagesModule 10 Electric CurrentangelineNo ratings yet
- CLS Aipmt 19 20 XII Phy Study Package 5 Level 2 Chapter 11Document30 pagesCLS Aipmt 19 20 XII Phy Study Package 5 Level 2 Chapter 11ShashvatNo ratings yet
- Physics Hewitt Chapter 2 AnswersDocument4 pagesPhysics Hewitt Chapter 2 AnswerssmeenaNo ratings yet
- Manometros Tabela de Conversao de Unidades de PressaoDocument1 pageManometros Tabela de Conversao de Unidades de PressaoRyber KalosNo ratings yet
- TM AHU 60R410A Onoff T SA NA 171205Document67 pagesTM AHU 60R410A Onoff T SA NA 171205Sam RVNo ratings yet
- Mechanics 2Document37 pagesMechanics 2You KumarNo ratings yet
- PFF260S 2023 Chapter 3.2 - Conservation Laws (Conservation of Energy)Document23 pagesPFF260S 2023 Chapter 3.2 - Conservation Laws (Conservation of Energy)glodis kamwanyaNo ratings yet
- Practical Physics: October University For Modern Sciences and Arts Faculty of DentistryDocument83 pagesPractical Physics: October University For Modern Sciences and Arts Faculty of DentistryMahasen BayoumiNo ratings yet
- Cyclotron Project Explains Particle AcceleratorDocument12 pagesCyclotron Project Explains Particle AcceleratorKartikey GuptaNo ratings yet
- Iiic - CC: Unisonic Technologies Co., LTDDocument15 pagesIiic - CC: Unisonic Technologies Co., LTDNetsanet ShikurNo ratings yet
- Physics Notes (Midterm 2)Document21 pagesPhysics Notes (Midterm 2)William StewartNo ratings yet
- Science Probe 9 Cheat Sheet Ch9-11Document2 pagesScience Probe 9 Cheat Sheet Ch9-11derpNo ratings yet
- 5.2.1 Capacitors ADocument10 pages5.2.1 Capacitors AStuart LittleNo ratings yet
- Thermal Conductivity of Metals ExperimentDocument12 pagesThermal Conductivity of Metals ExperimentシャルマチラグNo ratings yet
- Calculo Densidad Del Aire Cipm-2007Document7 pagesCalculo Densidad Del Aire Cipm-2007jagegonzalezNo ratings yet
- 4PH0 2PR Que 20150612Document20 pages4PH0 2PR Que 20150612adNo ratings yet
- C09 Add Maths Answer Form 4Document18 pagesC09 Add Maths Answer Form 4LEE HUI WEN MoeNo ratings yet
- Cambridge IGCSE: PHYSICS 0625/42Document16 pagesCambridge IGCSE: PHYSICS 0625/42...No ratings yet
- DC Power SupplyDocument14 pagesDC Power Supplyrjjain07No ratings yet
- Surface Area of Cubes and Cuboids (Print)Document5 pagesSurface Area of Cubes and Cuboids (Print)Lionel RonaldoNo ratings yet
- JPGS Test Question PaperDocument8 pagesJPGS Test Question PaperSapNo ratings yet
- Physics Test Review QuestionsDocument1 pagePhysics Test Review QuestionsGisele PhaloNo ratings yet
- tutorialETD SolnsDocument17 pagestutorialETD SolnsAkshay JaiwaliaNo ratings yet
- Yield of ConcreteDocument19 pagesYield of ConcreteSuvranil BanerjeeNo ratings yet
- Heat Transfer Lab ManualDocument59 pagesHeat Transfer Lab ManualMarNo ratings yet
- Othe Gas LawsDocument20 pagesOthe Gas Lawsromavin guillermoNo ratings yet