Professional Documents
Culture Documents
Acute Multifocal Placoid Pigment Epitheliopathy
Uploaded by
remotmOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Acute Multifocal Placoid Pigment Epitheliopathy
Uploaded by
remotmCopyright:
Available Formats
Acute Multifocal Placoid Pigment Epitheliopathy and Central Nervous System Involvement
Nine New Cases and a Review of the Literature
Henry S. OHalloran, FRCSI,1 Joseph R. Berger, MD,2 William B. Lee, MD,1 Dennis M. Robertson, MD,3 Joseph A. Giovannini, MD,4 Gregory B. Krohel, MD,5 Roy J. Meckler, MD,6 John B. Selhorst, MD,7 Andrew G. Lee, MD,8 David A. Nicolle, MD,9 Justin ODay, MD10
Objective: The authors describe nine new cases of acute multifocal placoid pigment epitheliopathy (AMPPE) with associated central nervous system (CNS) involvement and permanent visual sequelae. The study includes a review of the literature and discussion of evaluation, management, and treatment options. Design: Retrospective, noncomparative case series. Participants: Nine patients were identied with AMPPE and CNS involvement in addition to 22 patients reviewed in the literature. Main Outcome Measures: A review of nine patients with AMPPE and CNS involvement was performed. Charts were reviewed for age, gender, preceding viral prodromes, visual acuity, ophthalmologic examination ndings, CNS ndings, and treatment. Results: Thirty-one patients (nine new patients) were diagnosed with AMPPE and various degrees of CNS involvement. Ages ranged from 8 to 54 years, with an average of 27 years. Twenty-one males (68%) and 10 females (32%) were identied. Eleven patients (35%) had antecedent viral illnesses. Visual acuity was variable and ranged from 20/20 to count ngers. The spectrum of CNS ndings ranged from headaches to sagittal sinus thrombosis. Conclusions: Acute multifocal placoid pigment epitheliopathy can be associated with CNS abnormalities and permanent visual decits. Neuroimaging, lumbar puncture, and cerebral angiography analysis provide useful diagnostic tools when CNS involvement is suspected. Intravenous corticosteroids and collaboration with neurovascular colleagues should be considered in these situations. In cases complicated by CNS arteritis, immunosuppressive agents can be a benecial adjunct to corticosteroids. Ophthalmology 2001;108:861 868 2001 by the American Academy of Ophthalmology. In Gasss1 original description of acute multifocal placoid pigment epitheliopathy (AMPPE) in 1968, he described the salient and diagnostic features of the disorder. The lesions in the retina were noted to be creamy white and were found at the level of the retinal pigment epithelium. They were also noted to be placoid and multifocal. The uorescein angiogram in one patient was described as showing early hypouorescence and late hyperuorescence, the classic features of this disorder. However, at that time, no description was made of central nervous system (CNS) involvement in these patients. Subsequently, 17 papers have described 22 cases of AMPPE with CNS involvement.218 The CNS involvement ranged in severity from persistent headaches to diffuse vasculitis leading to death.3,4 We identied nine additional patients of AMPPE with various degrees of CNS involvement ranging from headaches to cerebral infarcts,
Originally received: November 9, 1999. Accepted: February 8, 2001. Manuscript no. 99731. 1 Department of Ophthalmology, University of Kentucky, Lexington, Kentucky. 2 Departments of Neurology and Internal Medicine, University of Kentucky, Lexington, Kentucky. 3 Department of Ophthalmology, Mayo Clinic, Rochester, Minnesota. 4 Department of Ophthalmology, Georgetown University, Washington, DC. 5 Private Practice, Troy, New York. 6 Private Practice, Louisville, Kentucky.
2001 by the American Academy of Ophthalmology Published by Elsevier Science Inc.
Departments of Ophthalmology and Neurology, Saint Louis University, St. Louis, Missouri. 8 Departments of Ophthalmology and Neurology, University of Iowa, Iowa City, Iowa. 9 Department of Ophthalmology, London University, London, Ontario, Canada. 10 Department of Ophthalmology, St. Vincents Hospital, Fitzroy, Victoria, Australia. The authors have no nancial interest related to this manuscript. Correspondence to Henry S. OHalloran, FRCSI, Department of Ophthalmology, University of Kentucky, Lexington, KY 40536. E-mail: hohall@pop.uky.edu.
ISSN 0161-6420/00/$see front matter PII S0161-6420(01)00565-6
861
Ophthalmology Volume 108, Number 5, May 2001
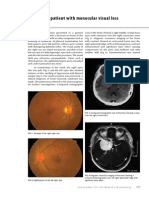
Figure 1. A uorescein angiogram of the left eye in early arteriovenous phase demonstrating areas of hypouorescence.
Figure 2. A uorescein angiogram of the left eye in late arteriovenous phase demonstrating areas of hyperuorescence.
and herein we discuss these patients in detail. We also reviewed the relevant literature on the subject and formulated a clinical plan for the evaluation, management, and treatment of patients with complicated AMPPE.124
Patients and Methods
A MEDLINE and Grateful Med search was performed for all references to AMPPE and retinitis, with and without viral prodromes. The search terms used included acute multifocal placoid pigment epitheliopathy and retinitis. Each of these papers was examined for references to AMPPE and CNS involvement. A total of 17 papers were identied. Subsequently, a letter of notice of our paper was sent to all members of The North American Neuroophthalmology Society listed on the societys mailing list. A total of 37 replies were received. From these replies, we identied eight other patients with AMPPE and CNS involvement. These are reported along with our own patient.
Patient 1
In April 1997, a 16-year-old white male sought treatment from his ophthalmologist after development of a scotoma in the left eye, which he related as a prodrome of his migraine, with the exception that it was noted to last longer than usual. On examination, he was noted to have a visual acuity of 20/20 in both eyes and chorioretinal lesions that demonstrated early hypouorescence and late hyperuorescence on uorescein angiography (Figs 1 and 2). He was started on prednisone 100 mg, which was tapered gradually over 2 weeks. One year later, the patient had a seizure. Magnetic resonance imaging (MRI) demonstrated meningeal enhancement and right frontal and parietal lobe encephalomalacia with areas of enhancement (Fig 3). An electroencephalogram (EEG) was performed that showed right frontal slowing with intermittent sharp waves. A diagnosis of viral meningitis was made, and the patient was hospitalized with a presumed diagnosis of herpes simplex encephalitis and treated with intravenous acyclovir. One month later, he sought treatment for severe headaches, confusion, and left-sided numbness. He reported no visual symptoms. Lumbar puncture analysis revealed an opening pressure of 396 mm of water, 28 white blood cells/high-power eld (100% mononuclear), protein 85 mg/dl, glucose 44 mg/dl, and immuno-
globulin G 5.7 mg/dl (normal range, 2.0 5.0 mg/dl). A repeat tapering dose of prednisone was administered beginning with a daily dose of 100 mg. A repeat MRI showed biparietal hemorrhages, and an angiogram showed a superior sagittal sinus thrombosis. A right parietal hemorrhage was surgically evacuated, and a brain biopsy was obtained in May 1998. The brain tissue appeared normal histologically. He was treated with phenytoin 400 mg daily and warfarin. After the prednisone taper was completed, dexamethasone 2 mg twice daily was added to the therapeutic regimen. A thorough hematologic evaluation revealed no evidence of coagulation disorder. Screenings for the presence of antinuclear antibodies, cytoplasmic and perinuclear antineutrophil cytoplasmic antibodies, rheumatoid factor, anti-Ro, and anti-La antibodies were all negative. The Westergren sedimentation rate was 5 mm/ hour. Two weeks after surgery, the patient was alert and orientated. The CNS examination revealed mild vibratory sensory loss chiey in his left lower extremity. Visual acuity was 20/30 in the left eye and 20/20 in the right eye. Dilated fundus examination revealed ovoid lesions in the periphery of both retinas consistent with resolved AMPPE. The rest of the eye examination was within normal limits. He had continued to take prednisone and phenytoin since the time of brain biopsy. No new neurologic or ophthalmo-
Figure 3. Magnetic resonance image (MRI) demonstrating a T1-weighted sagittal image showing right frontal (black arrowhead) and parietal encephalomalacia (black arrow) with enhancing borders and areas of meningeal enhancement.
862
OHalloran et al AMPPE and CNS Involvement
phosphamide in addition to the systemic corticosteroids. The corticosteroids were tapered, and the cyclophosphamide was gradually increased to 200 mg daily, at which time his condition improved. Six months after presentation to the ophthalmology department, his visual acuity was 20/20 in both eyes and he returned to work with no restrictions. He was no longer taking cyclophosphamide at that time. At a telephone follow-up in October 1998, the patient was stable with no new symptoms.
Patient 3
In July 1998, a 36-year-old white male sought treatment at the Department of Ophthalmology at Georgetown University for spots in front of his left eye that had lasted for 4 days and of his right eye that had lasted for 1 day. The patient described a viral-like illness that had developed 10 days previously. On examination, his visual acuity was 20/30 in the right eye and 20/50 in the left eye. There was a slight afferent pupillary defect in the left eye. The results of the slit-lamp examination were otherwise normal. A fundus examination showed multiple elevated creamy white plaques in both eyes, and the uorescein angiogram showed early hypouorescence with late hyperuorescence consistent with AMPPE. The patient began taking prednisone 40 mg daily. After 11 days, the patient was reexamined and visual acuity was unchanged in the right eye but had deteriorated to 20/200 in the left eye. Funduscopic examination was unchanged. Seven days later, the patient had an acute episode of left-sided numbness after he discontinued corticosteroids without medical consultation. His vision had deteriorated in the left eye to counting ngers. Corticosteroids were reinstituted at 40 mg per day orally. One week later, the patient sought treatment for persistent left-sided numbness present over the previous 7 days. He was hospitalized and started on methylprednisolone 1 g intravenously daily for 5 days. A lumbar puncture demonstrated an elevated protein of 88 mg/dl. An MRI showed multiple T2 signal abnormalities in the thalamus and in the periventricular white matter. The patient was discharged after 5 days of being administered prednisone with a gradual taper. At the follow-up examination 2 months after discharge, he was in good health except for some persistent left-sided numbness. Visual acuity was 20/20 in the right eye and 20/25 in the left eye. Fundus examination showed patchy areas of retinal pigment changes but no active lesions.
Figure 4. Fundus photograph of the right eye demonstrating multiple circumscribed chorioretinal lesions.
logic symptoms have been noted during the follow-up interval of 13 months.
Patient 2
A 25-year-old white male sought treatment from the Department of Ophthalmology at the Mayo Clinic in March 1993 with symptoms of blurred vision in the right eye. Four months before this visit he was diagnosed with viral meningitis and posterior uveitis. At that time, he was started on systemic corticosteroids with full resolution of his symptoms. One month later, while the corticosteroids were tapered, generalized malaise, headaches, a right hemiparesis, and difculty with bladder and bowel control developed. His symptoms improved on reinstitution of his corticosteroid therapy at 60 mg per day. At presentation to the ophthalmology department, his visual acuity was 20/40 in the right eye and 20/20 in the left eye. There were multiple circumscribed chorioretinal lesions in each eye, some associated with early pigment rarefaction and pigment clumping and others with a creamy discoloration (Figs 4 and 5). A uorescein angiogram showed patchy areas of hypouorescence associated with some of the lesions in the early part of the study and late hyperuorescence. These ndings were consistent with AMPPE. Magnetic resonance imaging studies of the head and cervical spine with and without gadolinium were negative. A four-vessel angiogram demonstrated evidence of cerebral arteritis. The patient began a daily oral dose of 50 mg of cyclo-
Patient 4
A 30-year-old white female sought treatment at the Albany Medical Center in December 1989, reporting decreased vision in both eyes over the preceding week. This was associated with a persistent headache that had lasted 1 week. On examination at presentation, her visual acuity was 20/70 in the right eye and 20/200 in the left eye. The pupils were xed, dilated, and unreactive to light secondary to pharmacologic iridoplegia. Fundus examination revealed placoid lesions consistent with AMPPE. The results of a computed tomography (CT) examination of the head at that time were normal. Over the next 3 weeks, the headache worsened and her visual acuity decreased to nger counting in both eyes. She was then admitted to the hospital. During hospitalization, she reported episodic ataxia and left-sided numbness. The results of a repeat CT, an MRI, and a four-vessel angiogram were normal. A lumbar puncture revealed a mild pleocytosis, elevated pressure of 270 mm of water, and normal protein and glucose levels. Fundus examination revealed papilledema in both eyes. She was administered 60 mg of prednisone, which prompted swift resolution of her headache. Her visual acuity failed to improve and the prednisone was increased to 80 mg daily. The visual acuity improved to 20/70 in the right eye and 20/200 in the left eye, but the
Figure 5. Fundus photograph of the left eye demonstrating multiple circumscribed chorioretinal lesions.
863
Ophthalmology Volume 108, Number 5, May 2001
papilledema worsened signicantly. The patient underwent a left optic nerve sheath fenestration, and her visual acuity improved to 20/50 in the right eye and 20/80 in the left eye. The prednisone was tapered gradually, and 3 months later her visual acuity was stable at 20/30 in both eyes. She remained stable over the next year, but problems with persistent elevated intracranial pressure subsequently developed that resolved with lumbar-peritoneal shunting. Over the following 7 years, she had no further recurrences of her AMPPE and her visual acuity is stable at 20/30 in the right eye and 20/20 in the left eye. Fundus examination showed some pigment mottling but no active lesions. Her headaches have fully resolved.
Patient 5
A 44-year-old white female with a history of AMPPE that had been documented 6 years previously was admitted to the Department of Neurology at the Jewish Hospital in Louisville in 1996 for investigation of migratory paresthesias sequentially involving her right side of face, right hand, and right biceps area that had persisted over a 2-month period. Her visual acuity at that time was 20/20 in both eyes, although she stated that her vision was subjectively worse in the right eye and had remained so since her episode of AMPPE. Humphrey 30 to 2 visual elds were normal, and fundus examination revealed some mild retinal pigment epithelial changes consistent with inactive AMPPE. On neurologic examination, the only abnormality elicited was some subjective decreased sensation on the right side of the face and the right arm. An MRI of the head revealed a small enhancing lesion at the left midbrainpontine junction and two smaller periventricular white matter T2 lesions. Cerebrospinal uid examination was remarkable for a mildly elevated IgG index of 0.77 with a mildly elevated synthesis rate of 5.2. Four weak oligoclonal bands were detected along with a normal myelin basic protein level at less than 1. A multiple sclerosis-like illness was suspected, and the patient received a dose pack of methylprednisolone starting at 60 mg orally daily with a gradual taper. Over the following 3 years, the patient received three further courses of intravenous methylprednisolone for progression of the T2-weighted lesions seen on MRI scanning. Her visual acuity at all times remained stable and there were no further episodes of AMPPE.
Figure 6. Fundus photograph of the right eye with multiple cream colored lesions at the level of the retinal pigment epithelium (black arrow).
Patient 7
A 27-year-old white female sought treatment at the Department of Ophthalmology at the Cullen Institute in 1996 for decreased vision in her left eye. She had a previous episode of the same problem in 1993, at which time she was told that she had a viral inammation in her retina, which was treated with oral prednisone and resolved without sequelae. In December 1996, she experienced an upper respiratory infection, and as this resolved she noted a sudden onset of painless ashing lights in her right eye. Over 3 days, she noticed decreased temporal vision in her right eye and a subjective decrease in her vision in the right eye. On examination, her visual acuity was 20/20 in both eyes. There was a trace afferent pupillary defect in the right eye. Visual elds showed a normal left eye, but the right eye showed an enlarged blind spot, which extended into the temporal periphery. Anterior segments were normal, but there was a mild vitritis in the right eye. Fundus examination showed several deep creamy colored lesions at the level of the retinal pigment epithelium in the right eye (Fig 6). Examination of the optic nerves showed minimal hyperemia of the right disc with some nasal swelling and a normal left disc. A diagnosis of AMPPE was made, but no treatment was instituted. Two years later she sought treatment for an episode of decreased vision in the left eye. She was treated by a neurologist with a dose pack of methylprednisolone and gradually improved. An MRI revealed optic nerve enhancement, multiple bilateral periventricular T2-weighted lesions, and an enlarged pituitary gland. Cerebrospinal uid examination at that time was normal, including the multiple sclerosis panel. The patient was then referred for an ophthalmologic consultation, at which time the visual acuity was 20/20 in the right eye and 20/25 in the left eye, and a mild left afferent pupillary defect was noted. Funduscopic examination of the left eye did not reveal any retinal lesions. There was no evidence of recurrence of AMPPE, and the patient received no treatment at this time. After 3 months, she stated that her vision had returned to normal.
Patient 6
A 39-year-old white female sought treatment at the Bethesda Eye Institute in St. Louis in 1992 for investigation of episodes of decreased peripheral vision in her right eye. These episodes lasted for approximately 20 minutes and had occurred six times in the previous 4 months. She also described an almost constant headache of varying severity. A CT examination performed at this time had negative results. On ophthalmic examination, her visual acuity was 20/20 in the right eye and 20/30 to 2 in the left eye. An afferent pupillary defect was present on the left eye. Funduscopic examination revealed mild optic nerve swelling in the left eye and several peripheral placoid lesions in both eyes, consistent with AMPPE. A repeat MRI showed a-right sided parasagittal contrast-enhancing lesion located to the right of the corpus callosum and straddling the graywhite matter junction. The results of a lumbar puncture were normal. The patient was given a dose pack of intravenous methylprednisolone and a follow-up taper of oral corticosteroids. Her headaches stopped shortly thereafter. Over 2 months, the disc edema lessened and visual acuity improved to 20/20 in the left eye. The afferent pupillary defect persisted, however. She was then lost to follow-up.
Patient 8
A 51-year-old white male was seen in the Eye Department at London Health Sciences Center in Ontario in 1997 for a routine eye examination. He had no new visual symptoms to report. Of note, the patient had a previous history of bilateral AMPPE complicated by cerebral vasculitis 18 months earlier and multiple old infarcts in the right temporal lobe consistent with strokes seen on
864
OHalloran et al AMPPE and CNS Involvement
Table 1. Clinical Data of Nine New Cases with Acute Multifocal Placoid Pigment Epitheliopathy and Central Nervous System Findings
Age Visual Acuity Patient (yrs) Gender before Treatment 1 2 3 4 5 6 7 8 9 16 25 36 30 38 39 27 51 49 M M M F F F F M M 20/20 both eyes 20/40; 20/20 20/30; 20/50 20/70; 20/200 20/40; 20/30 20/20; 20/30 20/20 both eyes Unknown 20/40; 20/50 Visual Acuity after Treatment 20/20; 20/30 20/20 both eyes 20/20; 20/25 20/30; 20/20 MRI ndings FL and PL encephalomalacia, ME, PL hemorrhages Normal Hyperintense signal abnormality in thalamus and PVWM Normal Treatment OS and BB SS and ISA OS and SS Central Nervous System Signs and Symptoms HA, confusion, scotoma, seizure, paresthesias HA, hemiparesis, incontinence Paresthesias, scotoma
OS, ONSF, LPS HA, ataxia, disc edema, paresthesias 20/20 both eyes Hyperintense signal abnormality OS and SS Paresthesias in midbrain pons junction and PVWM 20/20 both eyes Hyperintense signal abnormality OS and SS HA, disc edema of PVWM and PG enlargement 20/20; 20/25 Hyperintense signal abnormality OS Scotoma, disc edema of right corpus callosum 20/100 both eyes Right-sided TL infarcts OS and SS Quadrantanopia, paresthesias 20/20 both eyes Normal OS HA, confusion, dysphagia
BB brain biopsy; F female; FL frontal lobe; HA headache; ISA immunosuppressive agent; LPS lumbar peritoneal shunt; M male; ME meningeal enhancement; MRI magnetic resonance imaging; ONSF optic nerve sheath fenestration; OS oral steroids; PG pituitary gland; PL parietal lobe; PVWM periventricular white matter; SS systemic steroids; TL temporal lobe.
MRI. Visual acuity on examination was 20/100 in both eyes. Visual elds revealed a left superior homonymous quadrantanopia secondary to his cerebral infarcts. Fundus examination showed atrophic areas at the level of the retinal pigment epithelium, consistent with inactive AMPPE. The patient reported previous therapy with intravenous corticosteroids during the acute illness followed by a tapering dose of oral corticosteroids.
Patient 9
A 49-year-old white male sought treatment at the Eye Department at St. Vincents Medical Center in Victoria, Australia, in 1998. He reported a high fever over the preceding few days, along with myalgias and headaches. On ophthalmic examination, he had bilateral iritis and fundus lesions consistent with AMPPE. His visual acuity was 20/40 in the right eye and 20/50 in the left eye. A uorescein angiogram demonstrated the characteristic ndings of early hypouorescence and late hyperuorescence. The patient subsequently experienced a single episode of confusion and dysphasia in association with a high fever that lasted a few hours. Neuroimaging included negative CT and MRI examinations of the head. A presumed diagnosis of vasculitis was made, and the patient was treated with oral prednisone, after which his symptoms and fever resolved dramatically. At follow-up, he had no documented recurrences over a 10-month interval, and his vision returned to 20/20 in both eyes.
Discussion
Acute multifocal placoid pigment epitheliopathy is a welldescribed chorioretinal inammatory disease of uncertain origin. It usually occurs in both eyes simultaneously, but involvement can be delayed in the second eye. This condition manifests as yellow-white placoid lesions at the level of the retinal pigment epithelium, situated mainly in the posterior pole and rarely anterior to the equator. The lesions
tend to fade after a few days, and after 2 weeks they are replaced by partly depigmented pigment epithelium clumps. If the primary lesions do not involve the fovea, the visual prognosis tends to be good.1,17,25 The visual acuity can be as poor as counting ngers or as good as 20/20 during the acute illness. The worst individual recovery in visual acuity for males in the literature was 20/80; the worst individual recovery in vision for females in the literature was 20/ 200.131 Of our nine patients, the worst visual acuity was seen in patient 4, in whom the visual acuity was 20/200 in the left eye and 20/70 in the right eye (Table 1). Acute multifocal placoid pigment epitheliopathy uncomplicated by CNS involvement appears to affect males and females equally and has a predilection for young adults.25 However, in published cases of AMPPE with CNS involvement, 16 males have been described, whereas only six females have been discussed. In our case series, there were ve males and four females, which makes a total of 21 males and 10 females with documented AMPPE complicated by CNS involvement (Table 1). This difference may reect selection bias. The average age (where age was given) of all these cases was 27 years, with a range from 8 to 54 years. The cause is uncertain, but evidence is accumulating that indicates that the primary lesion is in the small choroidal arterioles and that secondary ischemic changes produce disruption of the pigment epithelium, resulting in typical placoid lesions.12,26 Indocyanine green angiography supports choroidal hypoperfusion as a likely mechanism of injury rather than primary pigment epitheliopathy.26,27 This ischemia leads to retinal pigment epithelial scarring without permanent retinal receptor damage, which explains why the visual prognosis is usually, but not invariably, good.1,25,26 The frequent occurrence of an antecedent febrile illness raises the question of an infectious agent as the trigger for
865
Ophthalmology Volume 108, Number 5, May 2001
this illness. A viral illness is frequently suspected, and one report documents a rising titer of adenovirus type 5 in a patient with active AMPPE.15,22,29 In fact, of the published cases of AMPPE with CNS ndings, 6 of 16 males (37.5%) and 1 of 6 females (16%) described an antecedent illness. Of our nine patients, four of the males (patients 1, 2, 3, and 9) and one of the females (patient 7) described a prodromal illness. In addition to viruses, tuberculosis, mycobacterium avium, and an allergic reaction to penicillin have all been implicated as the causative agents.1,19,31 These external agents can produce a toxic effect in one of three ways: (1) by direct toxic effect or infection of vascular tissues, (2) by immune mechanisms, (3) or by a combination of both.31 It is possible that these individuals have a genetic predisposition to vasculitis that is precipitated by introduction of an external antigen as a promoter of inammation. Wolf et al23 described a family in which a mother and son experienced optic neuritis and a daughter experienced AMPPE all within a 6-month period, suggesting a genetic predisposition; indeed, two of our patients (patients 4 and 7) had AMPPE and optic neuritis-type symptoms. A direct toxic effect of viruses on vascular tissue is seen in equine viral arteritis and immune factors predominate in Aleutian mink disease, in which viral specic immune complexes are found in vessel walls. The nding that only certain strains of mink are susceptible reinforces the theory of a genetic predisposition.32 In 1990, Wolf et al23 described a group of patients with HLA-B7 and HLA-DR2 antigens who had AMPPE. Other familial linkages have been described.33,34 Despite these associations, no denitive association has been proven between AMPPE, genetic predisposition, or any causative agent. Ocular pathologic characteristics of acute AMPPE are not available, and yet the association between AMPPE and various forms of vasculitis is assumed. In the previously documented cases, angiographic ndings consistent with vasculitis have been described.5,7,10,12,13 These ndings usually involve focal or diffuse vessel narrowing. Case 2 demonstrates diffuse vessel narrowing seen with vasculitis. In the one documented autopsy of a patient with AMPPE and CNS vasculitis, granulomatous cerebral arteritis was observed.4 Brewmeyer et al8 in 1993 performed a muscle biopsy on a patient, and it revealed arteriolar vasculitis, but other reported biopsies have been negative.11 The association between AMPPE and CNS complications has been well described. Of the 22 published cases, headaches were the most common symptom, occurring in 15 of the 22 patients (six females; nine males). Hemiplegia occurred in 5 of the 22 patients (two females; three males), and the rest had other neurologic signs. Of our nine patients, the signs included hemiparesis, dysarthria, numbness, ataxia, and incontinence. One of our patients (patient 1) had an associated superior sagittal sinus thrombosis, a neurologic complication not previously observed with AMPPE. Cerebral venous thrombosis is a recognized complication of CNS vasculitis.35,36 The seriousness of the CNS manifestations is highlighted by the fact that two patients have died of vasculitis. Therefore, it is important to have a high level of suspicion and a low tolerance for unusual symptoms or signs when dealing with AMPPE cases. Where a presenting systemic symptom was given in the published cases, it was most frequently headache (in 68%), followed by fever and malaise. In our cases, three patients had headaches at presentation, two with signs of meningeal irritation and two with a u-like illness. For these reasons, we suggest that all patients with AMPPE who have headache, fever, or vague meningeal signs at presentation be investigated for systemic and CNS vasculitis. In investigating these cases of suspected neurologic involvement, neuroimaging is very important. An MRI examination is considered superior to a CT examination because of its higher resolution and ability to identify subtle lesions. In the published cases, the most commonly seen lesions were hemorrhagic infarcts in the pons and the occipital lobes. Of our nine patients, six had positive MRI ndings, which included periventricular lesions in four, subtle corpus callosal lesions in one, and cerebral infarcts in one (Table 1). These lesions were better seen on T2-weighted imaging. For this reason, we recommend an MRI scan with and without contrast in all suspected cases of AMPPE with CNS involvement, with particular attention to the T2-weighted images. However, the results of MRI scans may be normal even in the presence of cerebral angiitis. A denitive diagnosis of angiitis requires cerebral angiography. Among the published cases, a lumbar puncture for collection of a sample of cerebrospinal uid was performed in 17 patients. Sixteen of these patients (94%) had an elevated white cell count, and 14 patients (82%) had increased protein levels. Six of the new patients whom we reported underwent cerebrospinal uid examination; the results of two were normal, two had an elevated white cell count, and three had an elevated protein level. Because a raised white cell count and protein level are strongly suggestive of CNS inammation, we recommend a lumbar puncture in all suspected cases of AMPPE with CNS involvement provided there are no contraindications. An EEG was performed only in one of our patients, and the results were mildly abnormal. In 6 of the 22 previously published cases of AMPPE with CNS involvement, an EEG was performed; the results of two were normal, whereas the others were read as abnormal without any specic localizing features. Although the EEG may show abnormalities, the benet in the diagnosis of cerebral vasculitis secondary to AMPPE is uncertain, and we do not routinely obtain this test. No diagnostic blood test exists for AMPPE. A sedimentation rate is considered to be a good indicator of generalized inammation, but in the published literature of AMPPE with CNS changes, only one of seven sedimentation rates was described as abnormal. Among our patients, only one sedimentation rate was recorded, and it too was normal. Thus, a sedimentation rate is not a useful adjunctive test in the diagnosis of CNS-complicated AMPPE. However, the presence of cellular casts in the urine is a good indicator of active AMPPE in the early stage of the disease.37 When CNS complications accompany AMPPE, treatment should be initiated rapidly. In a review of patients with primary angiitis of the CNS, Calabrese and Mallek31 reported a 95% mortality rate with no treatment, a 46% mortality rate with oral corticosteroid treatment alone, and
866
OHalloran et al AMPPE and CNS Involvement
an 8% mortality rate with corticosteroids and cytotoxic therapy. Although it cannot be presumed that primary angiitis of the CNS behaves the same as AMPPE accompanied by CNS complications, the combinations of AMPPE and CNS arteritis can lead to death. Consequently, AMPPE should be treated aggressively, and a team effort is often required to provide optimal management of these patients. Consultation with neurology and neurovascular colleagues can be valuable in treating these patients. We recommend treatment with intravenous corticosteroids followed by a slow oral steroid taper. A number of documented cases have shown that it can be difcult to taper off the steroids completely, and, in these cases, a switch to an immunosuppressive agent is recommended to decrease steroid-related effects.7 In patients with AMPPE complicated by CNS angiitis, we recommend treatment with intravenous corticosteroids and an immunosuppressive agent in combination. Our patients illustrate that AMPPE is not always a benign condition. It may, on occasion, represent a systemic inammatory disease with an initial and preferential ocular manifestation, and it can cause a generalized vasculitic condition with cerebral complications as well. The visual outcome, although usually good, can be poor, and the CNS effects can cause death. We recommend a high index of suspicion for CNS involvement. When CNS involvement is suspected, early neuroimaging and lumbar puncture will help establish the presence or absence of CNS involvement. In addition, cerebral angiography can provide evidence of arteritis. We emphasize that a team effort with neurovascular colleagues is essential in providing optimal patient treatment in cases where CNS involvement has been established. Aggressive treatment with intravenous corticosteroids should be considered, with a slow and gradual oral steroid taper thereafter. In cases of AMPPE complicated with CNS arteritis, intravenous corticosteroids in combination with an immunosuppressive agent such as cyclophosphamide may be necessary.
focal placoid pigment epitheliopathy with cerebral involvement. J Neurol Neurosurg Psychiatry 1991;54:779. Brewmeyer H, Nelles G, Huber M, et al. Pontine infarction in acute posterior multifocal placoid pigment epitheliopathy. J Neurol 1993;241:22 6. Fishman GA, Baskin M, Jednock N. Spinal uid pleocytosis in acute posterior multifocal placoid pigment epitheliopathy. Ann Ophthalmol 1977;9:33 6. Weinstein JM, Bresnick GH, Bell CL, et al. Acute posterior multifocal placoid pigment epitheliopathy associated with cerebral vasculitis. J Clin Neuroophthalmol 1988;8:195201. Bodiguel E, Benhamou A, Le Hoang P, Gautier JC. Infarctus ce re bral, e pithe liopathie en plaques et sarco dose. Rev Neurol (Paris) 1992;148:746 51. Holt WS, Regan CDJ, Trempe C. Acute posterior multifocal placoid pigment epitheliopathy. Am J Ophthalmol 1976;81: 40312. Sigelman J, Behrens M, Hilal S. Acute posterior multifocal placoid pigment epitheliopathy associated with cerebral vasculitis and homonymous hemianopia. Am J Ophthalmol 1979; 88:919 24. Bullock JD, Fletcher RL. Cerebrospinal uid abnormalities in acute posterior multifocal placoid pigment epitheliopathy. Am J Ophthalmol 1977;84:459. Ryan SJ, Maumenee AE. Acute posterior multifocal placoid pigment epitheliopathy. Am J Ophthalmol 1972;74:1066 74. Kersten DH, Lessell S, Carlow TJ. Acute posterior multifocal placoid pigment epitheliopathy and late-onset meningo-encephalitis. Ophthalmology 1987;94:393 6. Comu S, Verstraeten T, Rinkoff JS, Busis NA. Neurological manifestations of acute posterior multifocal placoid pigment epitheliopathy. Stroke 1996;27:996 1001. Kirkham TH, ffytche TJ, Sanders MD. Placoid pigment epitheliopathy with retinal vasculitis and papillitis. Br J Ophthalmol 1972;56:875 80. Jenkins RB, Savino PJ, Pilkerton AR. Placoid pigment epitheliopathy with swelling of the optic disks. Arch Neurol 1973;29:204 5. Savino PJ, Weinberg RJ, Yass n JG, Pilkerton AR. Diverse manifestations of acute posterior multifocal placoid pigment epitheliopathy. Am J Ophthalmol 1974;77:659 62. Van Buskirk EM, Lessell S, Friedman E. Pigmentary epitheliopathy and erythema nodosum. Arch Ophthalmol 1971;85: 369 72. Azar P Jr, Gohd RS, Waltman D, Gitter KA. Acute posterior multifocal placoid pigment epitheliopathy associated with an adenovirus type 5 infection. Am J Ophthalmol 1975;80: 10035. Wolf MD, Folk JC, Goeken NE. Acute posterior multifocal placoid pigment epitheliopathy and optic neuritis in a family. Am J Ophthalmol 1990;110:89 90. Annesley WH, Tomer TL, Shields JA. Multifocal placoid pigment epitheliopathy. Am J Ophthalmol 1973;76:511 8. Jones NP. Acute posterior multifocal placoid pigment epitheliopathy [review]. Br J Ophthalmol 1995;79:384 9. Deutmann AF, Oosterhuis JA, Boen-Tan TN, Aan de Kerk AL. Acute posterior multifocal placoid pigment epitheliopathy. Pigment epitheliopathy or choriocapillaritis. Br J Ophthalmol 1972;56:86374. Dhaliwal RS, Maguire AM, Flower RW, Arribas NP. Acute posterior multifocal pigment epitheliopathy. An indocyanine green angiographic study. Retina 1993;13:31725. Howe LJ, Woon H, Graham EM, et al. Choroidal hypoperfusion in acute posterior multifocal placoid pigment epitheliopathy. An indocyanine green angiography study. Ophthalmology 1995;102:790 8.
8. 9. 10. 11. 12. 13.
14. 15. 16. 17. 18. 19. 20. 21.
References
1. Gass JDM. Acute posterior multifocal placoid pigment epitheliopathy. Arch Ophthalmol 1968;80:177 85. 2. Spaide RF, Yannuzzi LA, Slakter J. Choroidal vasculitis in acute posterior multifocal placoid pigment epitheliopathy[published erratum appears in Br J Ophthalmol 1992;76:128]. Br J Ophthalmol 1991;75:6857. 3. Hammer ME, Grizzard WS, Travies D. Death associated with acute multifocal, placoid pigment epitheliopathy. Case report. Arch Ophthalmol 1989;107:170 1. 4. Wilson CA, Choromokos EA, Sheppard R. Acute posterior multifocal placoid pigment epitheliopathy and cerebral vasculitis. Arch Ophthalmol 1988;106:796 800. 5. Smith CH, Savino PJ, Beck RW, et al. Acute posterior multifocal placoid pigment epitheliopathy and cerebral vasculitis. Arch Neurol 1983;40:48 50. 6. Frohman LP, Klug R, Bielory L, et al. Acute posterior multifocal placoid pigment epitheliopathy with unilateral retinal lesions and bilateral disk edema. Am J Ophthalmol 1987;104: 548 55. 7. Stoll G, Reiners K, Schwartz A, et al. Acute posterior multi-
22.
23. 24. 25. 26.
27. 28.
867
Ophthalmology Volume 108, Number 5, May 2001
29. Fitzpatrick PJ, Robertson DM. Acute posterior multifocal placoid pigment epitheliopathy. Arch Ophthalmol 1973;89: 373 6. 30. Bird AC, Hamilton AM. Placoid pigment epitheliopathy. Presenting with bilateral serous retinal detachment. Br J Ophthalmol 1972;56:881 6. 31. Calabrese LH, Mallek JA. Primary angiitis of the central nervous system. Report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine (Baltimore) 1987;67:20 39. 32. Henson JB, Crawford TB. The pathogenesis of virus-induced arterial diseaseAleutian disease and equine viral arteritis. Adv Cardiol 1974;13:18391. 33. Wolf MD, Folk JC, Panknen CA, Goeken NE. HLA-B7 and HLA-DR2 antigens and acute posterior multifocal placoid pigment epitheliopathy. Arch Ophthalmol 1990;108:698 700. Kim RY, Holz FG, Gregor Z, Bird AC. Recurrent acute multifocal placoid pigment epitheliopathy in two cousins. Am J Ophthalmol 1995;119:660 2. Vidailhet M, Piette JC, Wechsler B, et al. Cerebral venous thrombosis in systemic lupus erythematosus. Stroke 1990;21: 1226 31. Mickle JP, McLennon JE, Lidden CW. Cortical vein thrombosis in Wegeners granulomatosis. Case report. J Neurosurg 1977;46:248 51. Priluck IA, Robertson DM, Buettner H. Acute posterior multifocal placoid pigment epitheliopathy. Urinary ndings. Arch Ophthalmol 1981;99:1560 2.
34.
35.
36.
37.
868
You might also like
- A Patient With Monocular Visual Loss: Pictorial MedicineDocument2 pagesA Patient With Monocular Visual Loss: Pictorial MedicineJennifer RodriguezNo ratings yet
- Alsberg 1 s2.0 S2451993621002279 MainDocument5 pagesAlsberg 1 s2.0 S2451993621002279 MainDoug PulaNo ratings yet
- Commentary: The Optic Neuritis Treatment TrialDocument2 pagesCommentary: The Optic Neuritis Treatment TrialEmmanuel Zótico Orellana JiménezNo ratings yet
- Clinical and Pathological Features of Different Types of LeprosyDocument10 pagesClinical and Pathological Features of Different Types of LeprosyRobert ThiodorusNo ratings yet
- Review of Optic Neuritis Treatment TrialDocument5 pagesReview of Optic Neuritis Treatment TrialsatrianiNo ratings yet
- Neurosyphilis Masquerading As An Acute Adie's Tonic Pupil: Report of A CaseDocument6 pagesNeurosyphilis Masquerading As An Acute Adie's Tonic Pupil: Report of A CaseOlvaria MisfaNo ratings yet
- Orbital Apex Syndrome from Herpes Zoster OphthalmicusDocument4 pagesOrbital Apex Syndrome from Herpes Zoster OphthalmicusYosiita KartinaaNo ratings yet
- Brain Abscess Potentially Secondary To Odontogenic Infection: CaseDocument4 pagesBrain Abscess Potentially Secondary To Odontogenic Infection: Caseلقمان حنفيNo ratings yet
- Belajar Diagnosa Mata TCM 03Document11 pagesBelajar Diagnosa Mata TCM 03fauzan pintarNo ratings yet
- Association of Myasthenia Gravis and Behçet's Disease: A CaseDocument4 pagesAssociation of Myasthenia Gravis and Behçet's Disease: A CaseTrần Văn ĐệNo ratings yet
- Vogt Koyanagi Harada SyndromeDocument9 pagesVogt Koyanagi Harada SyndromeBianca PavelNo ratings yet
- The Neurology of Eclampsia: Some Observations: Views and ReviewsDocument8 pagesThe Neurology of Eclampsia: Some Observations: Views and ReviewsFajar Rudy QimindraNo ratings yet
- Masquerade SyndromesDocument10 pagesMasquerade Syndromestony_chrisNo ratings yet
- Herpes Zoster OphthalmicusDocument12 pagesHerpes Zoster OphthalmicusGio Vano NaihonamNo ratings yet
- A Pain in The Butt - A Case Series of Gluteal Compartment Syndrome - PMCDocument6 pagesA Pain in The Butt - A Case Series of Gluteal Compartment Syndrome - PMCchhabraanNo ratings yet
- Clinical Reasoning: Rare Cause of Hemiparesis and Ataxia in A 36-Year-Old ManDocument8 pagesClinical Reasoning: Rare Cause of Hemiparesis and Ataxia in A 36-Year-Old MansamuelNo ratings yet
- American Journal of Ophthalmology Case Reports: Yael Sharon, David S. Chu TDocument5 pagesAmerican Journal of Ophthalmology Case Reports: Yael Sharon, David S. Chu TDaviel Quin DavNo ratings yet
- Orbital Apex Syndrome With Encephalitis A Rare and Serious Complication of Herpes Zoster Opthalmicus - Ludwig Melino Tjokrovonco 2Document12 pagesOrbital Apex Syndrome With Encephalitis A Rare and Serious Complication of Herpes Zoster Opthalmicus - Ludwig Melino Tjokrovonco 2Handi KrisnaniNo ratings yet
- Medicine: Refractory Hyponatremia in Neuromyelitis Optica in A Pediatric PatientDocument4 pagesMedicine: Refractory Hyponatremia in Neuromyelitis Optica in A Pediatric PatientMesan KoNo ratings yet
- Warumpornlimuntachai Medical Student in Emergency Department, Ramathibodi Hospital, Bangkok, ThailandDocument13 pagesWarumpornlimuntachai Medical Student in Emergency Department, Ramathibodi Hospital, Bangkok, ThailandAanam FathimaNo ratings yet
- JurnalDocument9 pagesJurnalMelita Amalia AyubaNo ratings yet
- (10920684 - Neurosurgical Focus) Mechanisms of Visual Loss in PapilledemaDocument8 pages(10920684 - Neurosurgical Focus) Mechanisms of Visual Loss in PapilledemaYusuf BrilliantNo ratings yet
- An Old Friend Revisited: Chloramphenicol Optic Neuropathy: Case ReportDocument3 pagesAn Old Friend Revisited: Chloramphenicol Optic Neuropathy: Case ReportckeshavaNo ratings yet
- Acute Hypotonia in An Infant (2017)Document3 pagesAcute Hypotonia in An Infant (2017)nikos.alexandrNo ratings yet
- Challenging Treatment of Bilateral Multiple Infection Panuveitis in HIV/AIDS PatientsDocument5 pagesChallenging Treatment of Bilateral Multiple Infection Panuveitis in HIV/AIDS PatientsRosyid PrasetyoNo ratings yet
- Visual Field Defects in Vascular Lesions The Lateral GeniculateDocument4 pagesVisual Field Defects in Vascular Lesions The Lateral GeniculateMarion FernandaNo ratings yet
- Anti-Myelin Oligodendrocyte Glycoprotein (MOG) Antibodies in Patients With Optic and SeizuresDocument17 pagesAnti-Myelin Oligodendrocyte Glycoprotein (MOG) Antibodies in Patients With Optic and SeizuresEmmanuel AguilarNo ratings yet
- Encefalitis Autoinmune 2011 PDFDocument11 pagesEncefalitis Autoinmune 2011 PDFDiana Vanessa SuarezNo ratings yet
- Basic Immunology 3rd EdiDocument5 pagesBasic Immunology 3rd EdiUpendra KotkondawarNo ratings yet
- JAMA - A Child With A White Pupil (Retinoblastoma)Document2 pagesJAMA - A Child With A White Pupil (Retinoblastoma)Rafael Carrillo-BaylonNo ratings yet
- Practice Parameter The Role of Corticosteroids in The Management of Acute Monosymptomatic Optic NeuritisDocument9 pagesPractice Parameter The Role of Corticosteroids in The Management of Acute Monosymptomatic Optic Neuritissatyagraha84No ratings yet
- Visual Evoked Potentials in Guillain-Barré Syndrome: MethodsDocument6 pagesVisual Evoked Potentials in Guillain-Barré Syndrome: MethodsYulianti PurnamasariNo ratings yet
- Acute Lymphoblastic Leukemia With Hypereosinphilia in A Child 2018Document8 pagesAcute Lymphoblastic Leukemia With Hypereosinphilia in A Child 2018David MerchánNo ratings yet
- 01 WNL 0000042787 51461 d1Document2 pages01 WNL 0000042787 51461 d1veronikiNo ratings yet
- Clinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945Document13 pagesClinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945aileenzhongNo ratings yet
- Reversible Ocular Toxicity Related To Tamoxifen Therapy: DE) Also of ofDocument3 pagesReversible Ocular Toxicity Related To Tamoxifen Therapy: DE) Also of ofsafasayedNo ratings yet
- Recurrent Optic Neuritis As A Clinical Manifestation in Multiple Sclerosis A Case Report - Anisa Feby ArifaniDocument11 pagesRecurrent Optic Neuritis As A Clinical Manifestation in Multiple Sclerosis A Case Report - Anisa Feby Arifanidenny070111209No ratings yet
- Posterior Reversible Encephalopathy Syndrome (PRES) : Electroencephalographic Findings and Seizure PatternsDocument7 pagesPosterior Reversible Encephalopathy Syndrome (PRES) : Electroencephalographic Findings and Seizure PatternsJasper CubiasNo ratings yet
- Of Optic Gliomas: ManagementDocument8 pagesOf Optic Gliomas: ManagementUtama Hadiputra SurbaktiNo ratings yet
- Laporan Kasus NeurooftalmologiDocument12 pagesLaporan Kasus NeurooftalmologiPradistya Syifa YudiasariNo ratings yet
- Algoritmo diagnostico de la ceguera monocular en una paciente con neurosarcoidosisDocument8 pagesAlgoritmo diagnostico de la ceguera monocular en una paciente con neurosarcoidosisFarid Santiago Abedrabbo LombeydaNo ratings yet
- Journal of Medical Case ReportsDocument4 pagesJournal of Medical Case ReportsSri Wahyuni YkNo ratings yet
- Miller Fisher Syndrome Mimicking Ocular Myasthenia GravisDocument10 pagesMiller Fisher Syndrome Mimicking Ocular Myasthenia GravisMonica Lauretta Sembiring IINo ratings yet
- Anti-N-methyl-D-aspartate Receptor (NMDAR) Encephalitis: A Series of Ten Cases From A University Hospital in MalaysiaDocument6 pagesAnti-N-methyl-D-aspartate Receptor (NMDAR) Encephalitis: A Series of Ten Cases From A University Hospital in MalaysiaAbhishek KayalNo ratings yet
- Topical TreatmentDocument7 pagesTopical TreatmentranpssNo ratings yet
- American Journal of Emergency Medicine: Dietrich Jehle Mary Claire Lark, Clay O'BrienDocument3 pagesAmerican Journal of Emergency Medicine: Dietrich Jehle Mary Claire Lark, Clay O'BrienGufront MustofaNo ratings yet
- Craniopharyngioma-The Anesthetic, Critical Care and Syrgical Challenges in The Managemnet of CPNDocument5 pagesCraniopharyngioma-The Anesthetic, Critical Care and Syrgical Challenges in The Managemnet of CPNpitriaNo ratings yet
- Syringomyelia in Neuromyelitis Optica Seropositive For Aquaporin-4 Antibody: A Case ReportDocument4 pagesSyringomyelia in Neuromyelitis Optica Seropositive For Aquaporin-4 Antibody: A Case ReportIJAR JOURNALNo ratings yet
- Parinaud Syndrome: Any Clinicoradiological Correlation?: L. Pollak - T. Zehavi-Dorin - A. Eyal - R. Milo - R. Huna-BaronDocument6 pagesParinaud Syndrome: Any Clinicoradiological Correlation?: L. Pollak - T. Zehavi-Dorin - A. Eyal - R. Milo - R. Huna-BaronJessica HerreraNo ratings yet
- Tacrolimus Optic NeuropathyDocument7 pagesTacrolimus Optic NeuropathyVlady BordaNo ratings yet
- Sindrom GradenigoDocument5 pagesSindrom GradenigoKelompok F8 CoasNo ratings yet
- Clinical Guideline: Management of The First Unprovoked Epileptic Seizure in Adults and ChildrenDocument6 pagesClinical Guideline: Management of The First Unprovoked Epileptic Seizure in Adults and Childrennaili nsnNo ratings yet
- Atypical Presentation of Optic Neuritis in Multiple Sclerosis - Dian ParamitasariDocument15 pagesAtypical Presentation of Optic Neuritis in Multiple Sclerosis - Dian ParamitasariGufront MustofaNo ratings yet
- Case Report: A Case of Herpes Simplex Virus-1 Encephalitis From A Medicolegal Point of ViewDocument4 pagesCase Report: A Case of Herpes Simplex Virus-1 Encephalitis From A Medicolegal Point of Viewzefri suhendarNo ratings yet
- Penetrating Keratoplasty in Active Acanthamoeba KeratitisDocument5 pagesPenetrating Keratoplasty in Active Acanthamoeba Keratitisdrvishalkulkarni2007No ratings yet
- 58 Neeta EtalDocument3 pages58 Neeta EtaleditorijmrhsNo ratings yet
- Ciliary Body Metastasis Masquerading As ScleritisDocument13 pagesCiliary Body Metastasis Masquerading As ScleritisDurgeshRastogiNo ratings yet
- Actionable Diagnosis of Neuroleptospirosis by Next-Generation SequencingDocument10 pagesActionable Diagnosis of Neuroleptospirosis by Next-Generation SequencingJorge AlvarezNo ratings yet
- Eyelid Ptosis Linked to Sinusitis in ChildDocument4 pagesEyelid Ptosis Linked to Sinusitis in ChildFadhli Muhammad KurniaNo ratings yet
- Handbook of Clinical Electrophysiology of VisionFrom EverandHandbook of Clinical Electrophysiology of VisionMinzhong YuNo ratings yet
- Arch Ophthalmol 1991 YannuzziDocument6 pagesArch Ophthalmol 1991 YannuzziremotmNo ratings yet
- Squisis MacularDocument8 pagesSquisis MacularremotmNo ratings yet
- Arch Ophthalmol 2011 YehDocument4 pagesArch Ophthalmol 2011 YehremotmNo ratings yet
- Am J Ophthalmol 2015 SridharDocument12 pagesAm J Ophthalmol 2015 SridharremotmNo ratings yet
- Choroidal Granulomas in Sistemic SarcoidosisDocument8 pagesChoroidal Granulomas in Sistemic SarcoidosisremotmNo ratings yet
- Ajopht 1977 RushDocument5 pagesAjopht 1977 RushremotmNo ratings yet
- Am J Ophthalmol 2014 YuDocument13 pagesAm J Ophthalmol 2014 YuremotmNo ratings yet
- Am J Ophthalmol 2015 DansinganiDocument5 pagesAm J Ophthalmol 2015 DansinganiremotmNo ratings yet
- Am J Ophthalmol 2015 ChenDocument10 pagesAm J Ophthalmol 2015 ChenremotmNo ratings yet
- Am J Ophthalmol 2014 Rahimy-3Document10 pagesAm J Ophthalmol 2014 Rahimy-3remotmNo ratings yet
- AntioxidantDocument8 pagesAntioxidantremotmNo ratings yet
- AJOPHT 1978 PriluckDocument4 pagesAJOPHT 1978 PriluckremotmNo ratings yet
- Acta Ophthalmol Scand 2000 Feigl-1Document4 pagesActa Ophthalmol Scand 2000 Feigl-1remotmNo ratings yet
- Brief Reports Out - 2001Document39 pagesBrief Reports Out - 2001remotmNo ratings yet
- Brief Reports Fev - .2001pdfDocument30 pagesBrief Reports Fev - .2001pdfremotmNo ratings yet
- Reducing Wrong IOL Implantation in Cataract CareDocument5 pagesReducing Wrong IOL Implantation in Cataract CareremotmNo ratings yet
- A Randomized, Double-Masked Trial of Topical Ketorolac VersusDocument6 pagesA Randomized, Double-Masked Trial of Topical Ketorolac VersusremotmNo ratings yet
- Katarak Penyebab KebutaanDocument96 pagesKatarak Penyebab KebutaanIhsan HaidarNo ratings yet
- Artigo01 10 - 3928 - 1081597X - 20120921 - 07Document11 pagesArtigo01 10 - 3928 - 1081597X - 20120921 - 07remotmNo ratings yet
- An Analysis of Lesion Size and Location in Newly Diagnosed CyDocument7 pagesAn Analysis of Lesion Size and Location in Newly Diagnosed CyremotmNo ratings yet
- Artigo 02 1-S2.0-S0161642012003545-MainDocument6 pagesArtigo 02 1-S2.0-S0161642012003545-MainremotmNo ratings yet
- Oral Mifepristone For Chronic Central Serous.26Document9 pagesOral Mifepristone For Chronic Central Serous.26remotmNo ratings yet
- Spaide PDFDocument11 pagesSpaide PDFremotmNo ratings yet
- Kanker ServiksDocument17 pagesKanker Serviksvenia endah tamaraNo ratings yet
- ECmed Eval SheetDocument1 pageECmed Eval SheetSalie GuarinoNo ratings yet
- H&P Cheat SheetDocument3 pagesH&P Cheat SheetWilliam YangNo ratings yet
- Essay 5Document13 pagesEssay 5api-549025764No ratings yet
- Heart SoundDocument29 pagesHeart Sounddianpratiwirahim100% (1)
- Fetal Distress Case DiscussionDocument55 pagesFetal Distress Case DiscussionHafsah G.No ratings yet
- BAD Cryotherapy Update March 2018 - Lay Review March 2018Document4 pagesBAD Cryotherapy Update March 2018 - Lay Review March 2018Mehret TechaneNo ratings yet
- Defining Treatment Response, Remission, Relapse, and RecoveryDocument21 pagesDefining Treatment Response, Remission, Relapse, and Recovery주병욱No ratings yet
- Acute Respiratory Failure For StudentDocument41 pagesAcute Respiratory Failure For Studentapi-379952350% (4)
- An Approach To The Diagnostic Study On Annavaha Srotodusti in Urdwaga Amlapitta WSR To Oesophagogastroduodenoscopic ChangesDocument4 pagesAn Approach To The Diagnostic Study On Annavaha Srotodusti in Urdwaga Amlapitta WSR To Oesophagogastroduodenoscopic ChangesEditor IJTSRDNo ratings yet
- Diabetic Ketoacidosis in ChildrenDocument39 pagesDiabetic Ketoacidosis in ChildrenRichard SiahaanNo ratings yet
- Rectal Exam Skill SheetDocument1 pageRectal Exam Skill SheetMuhammed ElgasimNo ratings yet
- Epilepsy GuidelinesDocument94 pagesEpilepsy GuidelinesAyu Cliquers100% (1)
- Lester 2015Document7 pagesLester 2015Khumaira SantaNo ratings yet
- Zollinger-Ellison Syndrome (Gastrinoma)Document15 pagesZollinger-Ellison Syndrome (Gastrinoma)Huy QuangNo ratings yet
- Globemed FormDocument4 pagesGlobemed Formangeloriondo1217No ratings yet
- Tle 10Document49 pagesTle 10peepee poopooNo ratings yet
- Endometrial CancerDocument192 pagesEndometrial CancerAbhishek VijayakumarNo ratings yet
- My PCOSDocument54 pagesMy PCOSanees_aneessNo ratings yet
- Case Pres A1 SurgDocument14 pagesCase Pres A1 SurgWyen CabatbatNo ratings yet
- NitrofurantoinDocument3 pagesNitrofurantoinapi-3797941No ratings yet
- Measuring Dental DiseasesDocument19 pagesMeasuring Dental DiseasesmisdduaaNo ratings yet
- The Effectiveness of Health Programs in Barangay Quezon, Arevalo, Iloilo CityDocument30 pagesThe Effectiveness of Health Programs in Barangay Quezon, Arevalo, Iloilo CityuhrlizNo ratings yet
- A Laser Peripheral Iridotomy Would Most Likely Relieve AngleDocument2 pagesA Laser Peripheral Iridotomy Would Most Likely Relieve AngleGlaucoma UnhasNo ratings yet
- Bacterial Patterns and Antibiotic Resistance in Grade Two Diabetic UlcersDocument15 pagesBacterial Patterns and Antibiotic Resistance in Grade Two Diabetic UlcersNadya AbigailNo ratings yet
- Bipolar Affective Disorder: Captain DR Yujal Man Singh Neuropsychiatrist, Shree Birendra HospitalDocument15 pagesBipolar Affective Disorder: Captain DR Yujal Man Singh Neuropsychiatrist, Shree Birendra HospitalYujal Man SinghNo ratings yet
- Multiple Organ Failure As A Cause of DeaDocument6 pagesMultiple Organ Failure As A Cause of DeaEliza DiaconescuNo ratings yet
- Pathology, Classification, and Grading of Neuroendocrine Neoplasms Arising in The Digestive System - UpToDateDocument45 pagesPathology, Classification, and Grading of Neuroendocrine Neoplasms Arising in The Digestive System - UpToDatewipi112No ratings yet
- HemorrhoidectomyDocument5 pagesHemorrhoidectomydrnareshkumar3281100% (1)
- Journal Club: DR Meera Nandan 3 Year MD ClinicalyogaDocument54 pagesJournal Club: DR Meera Nandan 3 Year MD ClinicalyogaMeera NandanNo ratings yet