Professional Documents
Culture Documents
Lab 1
Uploaded by
Ezechi Papaz UcheCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lab 1
Uploaded by
Ezechi Papaz UcheCopyright:
Available Formats
Objective: Determining the electrical equivalent between heat energy in calories and electrical energy in joules.
Apparatus: joule heat calorimeter with a resistor heater attach to its end, thermometer , current sensor, a voltage probe, science workshop setup, connecting wires, switch, DC power supply, and science Workshop Interpharse. Theory: The experiment is achieved by finding the electrical energy dissipated when water is heated is heated by submerging a heating resistor in water and running current through the resistor. Also the heat absorbed by water (in calories) is determined. Using the law of conservation of energy, if there are no energy losses to surrounding, all the energy given off by the resistor should be absorbed by the water. The electrical energy delivered to the resistor is given as E=IVT (J) The resistor is immersed in a known amount of water to absorb the heat. The rise in temperature is measured. If ( kg of water is contained in a calorimeter of mass kg and of specific heat cal/ , and T is the rise in its temperature, the heat absorbed is the heat absorbed is Q= ( T) + ( T) calorie
Where m is the mass of water, c is the specific heat of water (1cal/g. ), and T is the change in temperature of the water. Procedure of Experiment setup: One terminal of the switch is connected to the positive terminal of DC power supply and the other terminal of the switch is connected in series with probe from the energy transfer-calorimeter, and the terminal A of the science workshop interpharse. Also probe from the energy transfer-calorimeter is connected to the positive terminal of the current sensor. The negative terminal of the current sensor is connected in series with the probe from terminal B of the science workshop interpharse, and the negative terminal of the DC power supply. The probe from the terminal A of the science workshop interpharse is connected to the side of the current sensor. Before the connections, the switch is left open and it is switch on when connection is completed.
Experiment Procedure:
1. 2. 3. 4. 5.
Measure and record the room temperature. Weigh the empty container, and record its mass. Fill the container to indicate water level, and record its mass. The container with water is transfer into the energy calorimeter. The apparatus is setup as stated below. Schematic of the Setup for the Joule Heat Study
DATA COLLECTION
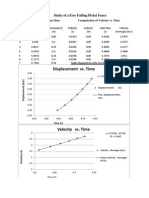
Study of Joule Heat Room temperature ( ) = Mass of calorimeter cup (g) = Mass of calorimeter cup +water = Mass of water (g) = 53.7 Initial temperature of water ( ) = Final temperature after heating it electrically ( Temperature Change ( =
Trial 1 20.0 24.8 80.8 56.0
Trial 2 20.0 24.8 78.5
18.0 22.5 4.5 276.552 4.935 0.443 474 1036.3 3.747
22.5 27.0 4.5 295.780 4.925 0.443 660 1440.0 4.868
=Tf-Ti=
Q = Heat absorbed by water (cal) = Average Voltage (V) = Average Current (A) = Total Time(s) = E = Electrical energy delivered (j) = Electrical Heat Equivalent = E/Q = Average E/Q= Expected Value Experimental % deviation 4.186j/cal 4.308 2.91%
4.308
Source of Experimental Error: 1. If temperature of the water in the container is higher than the room temperature before the experiment start, this will cause disparity in the date used to calculate the electrical heat equivalent needed for that mass of water. 2. When ice is added to the water is not allowed to completely melt, this would cause a much cooler temperature than expected at the specified voltages and current reading. 3. The present of ice in the container might skewed the mass of water since ice is less dense than water, this would also cause disparity in the data used to calculate the joules equivalent of electrical energy.
Conclusion: This experiment shows the relationship between the equivalent of electrical energy and heat energy using joule heat calorimeter with a resistor heater attach to its end. Post Lab Question: 1. Define the electrical equivalent of heat Answer: the electrical equivalent of heat is the amount of joules of electrical energy that are equivalent to one calorie of thermal energy. 2. In which unit of measure is it expressed? Answer: the unit is expressed in joules per kilocalorie. Since electrical energy is in joules and heat energy is kilocalories 3. What are the similarities and difference between the electrical equivalent of heat and the mechanical equivalent of heat? Similarities: both of them can be converted to order form energy e.g. they can be used to run turbine which generate electricity. Difference: the efficiency of converting electrical energy to thermal energy is always 100% but converting mechanical energy to thermal energy is less than 100%, this is because some of the efficiency was used to overcome friction. 4. If no heat is lost to the surrounding, what is the efficiency of converting electrical energy to thermal energy? Answer: Since there is no heat lost to the surroundings, the efficiency of converting electrical energy to thermal energy 100%
5.
What precaution should one observe to get the most accurate result?
Answer: a. b. In order to get accurate result, the water used should be clean from impurity and should also be either at the same temperature or one-two degree below the room temperature. When ice is added to the water , the water should be stirred properly until all ice is dissolved before using for experiment.
You might also like
- Mean-Free-Path Vs Pressure (Log - Log)Document1 pageMean-Free-Path Vs Pressure (Log - Log)Ezechi Papaz UcheNo ratings yet
- HW Chapter 9 SummaryDocument1 pageHW Chapter 9 SummaryEzechi Papaz UcheNo ratings yet
- Construct 95% CI for DataDocument1 pageConstruct 95% CI for DataEzechi Papaz UcheNo ratings yet
- Intramural Soccer RulesDocument4 pagesIntramural Soccer RulesEzechi Papaz UcheNo ratings yet
- Matlab Answer ToDocument2 pagesMatlab Answer ToEzechi Papaz UcheNo ratings yet
- HW#1Document1 pageHW#1Ezechi Papaz UcheNo ratings yet
- 3075 Hurricane Irene Alert - V1 (8-25)Document1 page3075 Hurricane Irene Alert - V1 (8-25)Ezechi Papaz UcheNo ratings yet
- f11 Midterm Study GuideDocument2 pagesf11 Midterm Study GuideEzechi Papaz UcheNo ratings yet
- 5 Plot of C Versus TDocument2 pages5 Plot of C Versus TEzechi Papaz UcheNo ratings yet
- Join The American Society For Mechanical Engineers (ASME) UMES SectionDocument2 pagesJoin The American Society For Mechanical Engineers (ASME) UMES SectionEzechi Papaz UcheNo ratings yet
- Assemble Program Reset Board Go To Terminal and Write "Load' Press Enter Go To Build Click Download Press Enter Write "G 2000"Document1 pageAssemble Program Reset Board Go To Terminal and Write "Load' Press Enter Go To Build Click Download Press Enter Write "G 2000"Ezechi Papaz UcheNo ratings yet
- Prime Package Clu PDFDocument4 pagesPrime Package Clu PDFVincentNo ratings yet
- 3075 Hurricane Irene Alert - V1 (8-25)Document1 page3075 Hurricane Irene Alert - V1 (8-25)Ezechi Papaz UcheNo ratings yet
- Dr. Singh ExcelDocument2 pagesDr. Singh ExcelEzechi Papaz UcheNo ratings yet
- Prime Package Clu PDFDocument4 pagesPrime Package Clu PDFVincentNo ratings yet
- AP Econ Chapter 11 questions reviewDocument4 pagesAP Econ Chapter 11 questions reviewEzechi Papaz UcheNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Static Exciter Aux Fed Vs Terminal FedDocument6 pagesStatic Exciter Aux Fed Vs Terminal Fedavikrisad100% (1)
- Planning Optical Power Budget in DWDM SystemsDocument48 pagesPlanning Optical Power Budget in DWDM Systemsrafael antonio padilla mayorgaNo ratings yet
- DH61DL TechProdSpec06Document88 pagesDH61DL TechProdSpec06Pio WalterNo ratings yet
- KP-38027 Maintenance Instructions A EnglishDocument34 pagesKP-38027 Maintenance Instructions A EnglishRonaldo GeradoresNo ratings yet
- Whirlpool PWA 830 A Service ManualDocument14 pagesWhirlpool PWA 830 A Service ManualIoana Ancuța MoiseNo ratings yet
- R7410210-Electrical Distribution SystemsDocument4 pagesR7410210-Electrical Distribution SystemssivabharathamurthyNo ratings yet
- Low Power Vlsi Design 2Document70 pagesLow Power Vlsi Design 2Rajesh PylaNo ratings yet
- GTP - LT AB Cable - CSPDCLDocument6 pagesGTP - LT AB Cable - CSPDCLNavya cablesNo ratings yet
- Pak Elektron Limited Technical Data Sheet of Transformer: SpecificationDocument1 pagePak Elektron Limited Technical Data Sheet of Transformer: SpecificationbilalNo ratings yet
- Tabhilashreddy (5 2)Document3 pagesTabhilashreddy (5 2)aravi173No ratings yet
- HPB PDL450 ManualDocument232 pagesHPB PDL450 ManualReinaldo Andres Castillo Jopia50% (2)
- Seco-DC-Drives Catalog en-US Rev2005Document40 pagesSeco-DC-Drives Catalog en-US Rev2005milacronNo ratings yet
- HPS Catalogue Transformer Products Web Version PDFDocument325 pagesHPS Catalogue Transformer Products Web Version PDFMoch Abduh AlcantaraNo ratings yet
- LCD TV: Owner's InstructionsDocument33 pagesLCD TV: Owner's Instructionsagrani1234No ratings yet
- Ansi C57.12.52Document18 pagesAnsi C57.12.52Jose DiazNo ratings yet
- 1734 Um018 - en e PDFDocument130 pages1734 Um018 - en e PDFFERNANDONo ratings yet
- Computer Graphics Unit1Document88 pagesComputer Graphics Unit1computerstudentNo ratings yet
- Design of A Novel DRFM Jamming System Based On AFB-SFBDocument5 pagesDesign of A Novel DRFM Jamming System Based On AFB-SFBHongdang DinhNo ratings yet
- Abit - M514 Schematics: Title Sheet ModifyDocument35 pagesAbit - M514 Schematics: Title Sheet ModifyThắng PhạmNo ratings yet
- Homework #05 (Phy 112) SolutionsDocument24 pagesHomework #05 (Phy 112) SolutionsKvn4N6No ratings yet
- Journeyman by Engr HerimeeDocument606 pagesJourneyman by Engr HerimeeHerimee TorenaNo ratings yet
- Testing Electronic ComponentsDocument98 pagesTesting Electronic Componentshajasoftware100% (3)
- Induction Motor Speed ControlDocument46 pagesInduction Motor Speed Controlsolo400060% (10)
- KP Test 5D Dual PI ENDocument4 pagesKP Test 5D Dual PI ENJorge Duran FidilioNo ratings yet
- Chevrolet Volt 5 Door Hatchback 2016 - 2021 First Responder Rescue SheetDocument2 pagesChevrolet Volt 5 Door Hatchback 2016 - 2021 First Responder Rescue Sheetfrancisco javierNo ratings yet
- Cooling Fans (Radiator Cooling Fan) - ALLDATA RepairDocument1 pageCooling Fans (Radiator Cooling Fan) - ALLDATA RepairFran Sanchez0% (1)
- Toshiba Power Supply ICs GuideDocument27 pagesToshiba Power Supply ICs Guideoscarcito08No ratings yet
- Job Sheet 1Document5 pagesJob Sheet 1Sue AzizNo ratings yet
- Perating Anual: VX-3200 O MDocument16 pagesPerating Anual: VX-3200 O MLisnik W. F. BerrielNo ratings yet
- ATX IO Implementations 11Document11 pagesATX IO Implementations 11周志杰No ratings yet