Professional Documents
Culture Documents
Investigating the vitamin C content of fruit juices
Uploaded by
Michael CollinOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Investigating the vitamin C content of fruit juices
Uploaded by
Michael CollinCopyright:
Available Formats
Activity 1.21 Is high C all it claims to be?
Purpose:
Write up
A1.21L CORE
To investigate the vitamin C content of fruit juice
The activity sheet presents a case that appeared in the news and this could be used to put the practical work in context. The students might investigate the vitamin C content of a range of different juices to determine which contain the highest level of the vitamin. To link more directly with the story on the activity sheet, students could test the vitamin C levels in the juice and compare the level with the quantity stated on the product. Note that the Edexcel GCE Biology specification requires you to know how to investigate the vitamin C content of food and drink. To test food, you have to mash it up with water and then carry out the test as for drinks.
Title: Investigating the vitamin C content of fruit juices Aim
To find out which of a range of different juices contains the most vitamin C, and calculate the percentage of vitamin C in each fruit juice.
Method
Use the Procedure below to record the method YOU used, in detail, including safety precautions taken, equipment used and reasons for your choice of equipment.
Procedure
You will need
0.1% DCPIP solution 1% vitamin C solution A range of fruit juices Test tubes Pipette to accurately measure 1cm3 Pipette or burette 1. Pipette 1 cm3 of 1% DCPIP solution into a test tube. 2. Using a pipette or burette, add 1% vitamin C solution drop by drop to the DCPIP solution. After adding each drop shake the tube gently. Continue to add drops of the vitamin C solution until the blue colour of the DCPIP has just disappeared. 3. Record the exact amount of the vitamin C solution that was added to decolourise the DCPIP solution. Repeat the procedure and average the result. 4. Repeat this procedure with the other fruit juices provided. If only one or two drops of the fruit juice decolourises the DCPIP, you could dilute the juice and repeat the test.
Activity 1.21 Is high C all it claims to be? Results
Record your results in a table. A sample table and results are shown below.
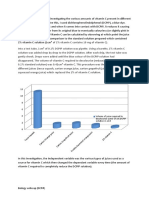
Sample results
Juice tested 1% vitamin C Grapefruit juice Pineapple juice Orange juice Orange drink Fresh lemon juice Bottled lemon juice Volume of juice required to decolourise 1 cm3 of 0.1% DCPIP solution/cm3 0.61 1.50 12.00 2.00 1.40 1.90 24.0 0.59 1.70 11.20 2.25 1.50 1.70 23.5 0.60 1.65 11.50 2.10 1.45 1.60 24.5
Write up
A1.21L CORE
Average of juice required /cm3 0.60 1.61 11.56 2.12 1.45 1.73 24.0
Vitamin C content of juice/mg cm3 10.0 3.8 0.5 2.8 4.1 3.5 0.25
The table gives the volume of various carton fruit juices that decolourised 1 cm3 of 0.1% DCPIP solution. The end point was when the blue tinge has completely disappeared. 0.6 cm3 of 1% vitamin C solution decolourised 1 cm3 of 0.1% DCPIP solution. 1 cm3 of the 1% vitamin C contains 10 mg of vitamin C so it takes 6 mg of vitamin C to decolourise 1 cm3 of DCPIP.
Conclusions
Use your results and the information above to work out how much vitamin C each of your fruit juices contained, in mg cm3. Discuss your findings and those shown in the sample results table with reference to your aim. Support your statements with evidence from your results, and relevant biological knowledge. Comment on any systematic or random errors in the data.
Evaluation
Propose any changes to the procedure that would improve the reliability and validity of the results.
You might also like
- A Level Biology A (SNAB) Core Practical 2 - Vitamin CDocument5 pagesA Level Biology A (SNAB) Core Practical 2 - Vitamin CBara' HammadehNo ratings yet
- Biology Core Practical 2Document6 pagesBiology Core Practical 2Roman Crame50% (2)
- Vitamin C content comparison of fresh vs processed juicesDocument14 pagesVitamin C content comparison of fresh vs processed juicesLee da DonNo ratings yet
- Activity 1.21 Vit C Report of Core Practical Edexcel AsDocument3 pagesActivity 1.21 Vit C Report of Core Practical Edexcel AsJesse EnglandNo ratings yet
- Bio ReportDocument8 pagesBio ReportTharshini_Indr_6713No ratings yet
- Measuring Vitamin C in Orange Juices (39 charactersDocument9 pagesMeasuring Vitamin C in Orange Juices (39 charactersSanngeeta100% (2)
- Vitamin C in Fruit JuicesDocument5 pagesVitamin C in Fruit JuicesANMOL journeyNo ratings yet
- Analysis of Concentration of Vitamin C IDocument21 pagesAnalysis of Concentration of Vitamin C IMahamud Hasan Prince100% (1)
- Vitamin CDocument15 pagesVitamin Czaiy67% (3)
- Vitamin C Write-UpDocument5 pagesVitamin C Write-UpannafiiNo ratings yet
- Investigating Vitamin C Concentration in Homemade and Store Bought SmoothiesDocument7 pagesInvestigating Vitamin C Concentration in Homemade and Store Bought SmoothiesisabelleNo ratings yet
- 2.2 Enzyme Concentration Core Practical Writing FrameDocument4 pages2.2 Enzyme Concentration Core Practical Writing FramepaligoddNo ratings yet
- Is High C All It Claims To BeDocument2 pagesIs High C All It Claims To BeYaw Brempong Yeboah100% (1)
- AS Biology Unit 3 Practical ExamDocument30 pagesAS Biology Unit 3 Practical ExamShamaNo ratings yet
- Observing MitosisDocument16 pagesObserving MitosisNur ShuhadaNo ratings yet
- DCPIP Write UpDocument2 pagesDCPIP Write UpHashim Chishty0% (1)
- Core Practical Experiments Unit 2: Root Tip SquashDocument9 pagesCore Practical Experiments Unit 2: Root Tip SquashHsia Ang100% (1)
- Temperature's Effect on Membrane PermeabilityDocument5 pagesTemperature's Effect on Membrane PermeabilityJett0% (1)
- Daphnia Write UpDocument3 pagesDaphnia Write UpASDFGHJKL9571% (7)
- Core Practical 4Document4 pagesCore Practical 4AyeshaNo ratings yet
- Vitmin C ReportDocument14 pagesVitmin C ReportOdongo TonnyNo ratings yet
- Chem Project - Class 12Document12 pagesChem Project - Class 12M AdithyaNo ratings yet
- DaphniaDocument15 pagesDaphniaSabila SiddiquiNo ratings yet
- Titration Chemistry Lab Report Vitamin CDocument4 pagesTitration Chemistry Lab Report Vitamin CAlias AliquidNo ratings yet
- Experiment On Vitamins - CONGSONDocument3 pagesExperiment On Vitamins - CONGSONShayne Angelique CongsonNo ratings yet
- CHEM 22161 Lab 1 :synthesis of Medicinal Agent: Synthesis of AspirinDocument8 pagesCHEM 22161 Lab 1 :synthesis of Medicinal Agent: Synthesis of AspirinKasun WekasingheNo ratings yet
- 1.vitamin C ContentDocument7 pages1.vitamin C ContentTheresa NgNo ratings yet
- Enzyme ConcentrationDocument16 pagesEnzyme ConcentrationSya Subi100% (1)
- Effects of Temperature and PH On The Enzymatic Activity of Salivary Amylase - JAENDocument12 pagesEffects of Temperature and PH On The Enzymatic Activity of Salivary Amylase - JAENCara JaenNo ratings yet
- Biology November ReportDocument3 pagesBiology November ReportIndrani GoswamiNo ratings yet
- CHEMISTRY ProjectDocument17 pagesCHEMISTRY ProjectMerin MariamNo ratings yet
- Analysis of Vitamin C in Fruit JuiceDocument17 pagesAnalysis of Vitamin C in Fruit JuiceManav RajeshNo ratings yet
- Determination of Vitamin CDocument7 pagesDetermination of Vitamin Capi-487208181No ratings yet
- Observing Mitosis in Garlic Roots (40Document14 pagesObserving Mitosis in Garlic Roots (40Muhammad Marsaid Bin KardiNo ratings yet
- Enzymes Report Biology AS Core Practical Write Up EdexcelDocument7 pagesEnzymes Report Biology AS Core Practical Write Up EdexcelthariniNo ratings yet
- List of Core Practicals For Edexcel Biology As Exam 2010Document35 pagesList of Core Practicals For Edexcel Biology As Exam 2010moalna80% (25)
- Effect of Alcohol on Membrane PermeabilityDocument2 pagesEffect of Alcohol on Membrane PermeabilityOrangesPlzNo ratings yet
- Enzyme Concentration and Activity Core PracticalDocument3 pagesEnzyme Concentration and Activity Core PracticalAgnes Okanlawon50% (2)
- CellsDocument20 pagesCellsppttppNo ratings yet
- Sds Page: Objective: TheoryDocument7 pagesSds Page: Objective: TheoryMuskan BishtNo ratings yet
- A Level Biology A Core Practical 16 - Rate of RespirationDocument8 pagesA Level Biology A Core Practical 16 - Rate of RespirationAlfred SangNo ratings yet
- DNAxtractn:StrawberryDocument3 pagesDNAxtractn:StrawberryVicki A.100% (1)
- Experiment 6.2Document3 pagesExperiment 6.2cindy_lee_11No ratings yet
- Vitamin C content in foods and drinksDocument2 pagesVitamin C content in foods and drinksTiffany :DNo ratings yet
- Vitamin C Content in Fruit JuicesDocument2 pagesVitamin C Content in Fruit JuicesSharifah Nurain100% (3)
- MEASURING VITAMIN CDocument3 pagesMEASURING VITAMIN CJohnNo ratings yet
- Vitamin C Content in Fruit JuicesDocument2 pagesVitamin C Content in Fruit JuicesRaja Marina IraniNo ratings yet
- Chem Soft Copy FinalDocument17 pagesChem Soft Copy FinalArvin KumarNo ratings yet
- Which Type of Fruit Juice Provides The Most Vitamin C?Document5 pagesWhich Type of Fruit Juice Provides The Most Vitamin C?Aswathy BijuNo ratings yet
- The Vitamin C Content of Fruit JuiceDocument16 pagesThe Vitamin C Content of Fruit JuiceTootsie87% (39)
- Biology Plan and Design Vitamin CDocument4 pagesBiology Plan and Design Vitamin CEliana SomanNo ratings yet
- Practical 2Document2 pagesPractical 2Dr.CharinNo ratings yet
- CP02 (1) 1Document2 pagesCP02 (1) 1ItsspongoNo ratings yet
- CPAC 2 - Vit CDocument3 pagesCPAC 2 - Vit CNoruwa EKUASENo ratings yet
- Vitamin C Content Comparison of Fresh vs Packaged Orange JuicesDocument7 pagesVitamin C Content Comparison of Fresh vs Packaged Orange Juicesgayatri BhargavaNo ratings yet
- Experiment of Vitamin: ProcedureDocument3 pagesExperiment of Vitamin: ProcedureMicah Joy MacalaladNo ratings yet
- Rohan Chavan Chem Investigatory ProjectDocument14 pagesRohan Chavan Chem Investigatory ProjectVedanta AswarNo ratings yet
- Food Sample Test For Procedure Observation InferenceDocument2 pagesFood Sample Test For Procedure Observation InferenceMismah Binti Tassa YanaNo ratings yet
- PKS A f4 VITAMIN CDocument4 pagesPKS A f4 VITAMIN CSK Pos Tenau100% (1)
- IAS Biology TRP1 CP2 StuDocument3 pagesIAS Biology TRP1 CP2 StuAyesha Gulzar0% (1)
- TextDocument3 pagesTextMichael CollinNo ratings yet
- QuestionsDocument1 pageQuestionsMichael CollinNo ratings yet
- Gecko document section 1 chemistry equationsDocument47 pagesGecko document section 1 chemistry equationsmartinskam94No ratings yet
- Hello WorldDocument1 pageHello WorldMichael CollinNo ratings yet
- Outline and Evaluate Explanations For INDEPENDENT BehaviourDocument2 pagesOutline and Evaluate Explanations For INDEPENDENT Behaviourtheone1998No ratings yet
- Nursing Hospital Staff Job Description AimsDocument20 pagesNursing Hospital Staff Job Description AimsSanthu Su100% (1)
- International Trade FinanceDocument2 pagesInternational Trade FinanceSadab RaeenNo ratings yet
- "Oral Application" Phase Collaborative ProjectDocument14 pages"Oral Application" Phase Collaborative ProjectAmparo CAMPOSNo ratings yet
- Census of India 2011: Village and Town Wise Primary Census Abstract for Lalitpur District, Uttar PradeshDocument198 pagesCensus of India 2011: Village and Town Wise Primary Census Abstract for Lalitpur District, Uttar PradeshShristi palNo ratings yet
- Computational Methods For Analyzing and Understanding Online CurrDocument140 pagesComputational Methods For Analyzing and Understanding Online Currjennifer sayongNo ratings yet
- The Enormous Crocodile: Alternative EndingsDocument2 pagesThe Enormous Crocodile: Alternative EndingsRania FarranNo ratings yet
- References For Peer Assessment in l2 Writing PosterDocument4 pagesReferences For Peer Assessment in l2 Writing Posterapi-537558603No ratings yet
- Advanced Audit and Assurance (AAA-INT) : Syllabus and Study GuideDocument23 pagesAdvanced Audit and Assurance (AAA-INT) : Syllabus and Study GuideFatima HasanNo ratings yet
- Federal Ethiopia Proclamation Protects EnvironmentDocument8 pagesFederal Ethiopia Proclamation Protects EnvironmentAnwar Endris100% (2)
- De Elderly Retirement Homes InPH Ver2 With CommentsDocument20 pagesDe Elderly Retirement Homes InPH Ver2 With CommentsgkzunigaNo ratings yet
- WHLP Week 5 Pen Antonio 2020 2021Document3 pagesWHLP Week 5 Pen Antonio 2020 2021Precious ArniNo ratings yet
- Self-Study Guide - Unit 7Document3 pagesSelf-Study Guide - Unit 7Daniela PachonNo ratings yet
- TodesDocument10 pagesTodesjxbalcazarNo ratings yet
- BASIC STUDY MANUAL Compiled From The Works of L. RON HUBBARDDocument83 pagesBASIC STUDY MANUAL Compiled From The Works of L. RON HUBBARDsirjsslutNo ratings yet
- Impact of Early Extraction of The Deciduous Canine On Relief of Severe Crowding Does It Influence Later Orthodontic InterventionsDocument6 pagesImpact of Early Extraction of The Deciduous Canine On Relief of Severe Crowding Does It Influence Later Orthodontic InterventionsRifqiyanti IsmiNo ratings yet
- DLL G9.PE.Q4.-week2Document6 pagesDLL G9.PE.Q4.-week2Konoha Maru100% (1)
- Objective: Calamba Bayside National Highschool Palingon Calamba CityDocument1 pageObjective: Calamba Bayside National Highschool Palingon Calamba CityKuhramaNo ratings yet
- Intro To Poetry Lesson PlanDocument3 pagesIntro To Poetry Lesson Planapi-383537126No ratings yet
- P Pretest and Posttest Phoenetic and MarungkoDocument25 pagesP Pretest and Posttest Phoenetic and MarungkoJellie Tamonan BarbajoNo ratings yet
- (The Language of Literature) Roger Fowler (Auth.) - The Language of George Orwell-Macmillan Education UK (1995) PDFDocument260 pages(The Language of Literature) Roger Fowler (Auth.) - The Language of George Orwell-Macmillan Education UK (1995) PDFPetr Škaroupka100% (2)
- 6 Hats TwoDocument29 pages6 Hats TwoDeepthi .BhogojuNo ratings yet
- Corporate Social Responsibility-27AprDocument23 pagesCorporate Social Responsibility-27AprDeepu JoseNo ratings yet
- POM NotesDocument131 pagesPOM NotesShwetha V N0% (1)
- Nwu Lesson Plan FormatDocument1 pageNwu Lesson Plan Formatapi-371231768No ratings yet
- PsaDocument71 pagesPsaKristina De los ReyesNo ratings yet
- Valdez - Worksheet 4 1Document2 pagesValdez - Worksheet 4 1Princess Dianne EstebanNo ratings yet
- District Health Management Information Systems (D-HMIS) - Tanzania - IICDDocument4 pagesDistrict Health Management Information Systems (D-HMIS) - Tanzania - IICDAbebe BelachewNo ratings yet
- Cover Letter CitiDocument11 pagesCover Letter CitiTheBusinessInsider100% (3)
- Probability Based Load Criteria For Structural Design PDFDocument6 pagesProbability Based Load Criteria For Structural Design PDFJessica CastriciniNo ratings yet