Professional Documents
Culture Documents
Hexamine Production Technology Guide
Uploaded by
Vinh Do ThanhOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Hexamine Production Technology Guide
Uploaded by
Vinh Do ThanhCopyright:
Available Formats
Hexamine Production Technology
Hexamine, short for hexamethylenetetramine, another name: H promoting agent;
molecular formula: (CH2 )6 N4 ; molecular weight140.19; chemical structure:
Hexamine has a wide array of applications in various fields. It is used as hardener
in synthetic resin, curing agent in phenolic plastic, catalyst in amino plastic, vulcanization promoter (H) in rubber industry and anti-shrinkage agent in textile industry. Also it is employed as diuretic agent in pharmaceutics, disinfectant in food industry and raw material for synthesizing many amino compounds, e.g., pesticide in agriculture. Besides, mixed with caustic soda and phenol naphthalene, it can be used as the absorption agent of phosgene for anti-toxic mask; after nitrification, it can be applied to make strong explosives.
Section A. Hexamine Properties
Hexamine is a white color crystal shape powders, odorless with slightly sweet
taste. It has anaphylactic irritation to the skin; specific gravity: 1.331(20 ); flash point: 250; stored in the air, it is stable, but easy to absorb moisture and lump. Hexamine is flammable with colorless flame. Its melting point is 263. When heated to 100 at normal pressure, minor part of it will be sublimated and decomposed into methylamine. Hexamine has a very good solubility in water. At 25, its concentration in saturated aquous solution is 46.5%. Hexamine is a weak alkaline reagent with PH value: 8-8.5. When ammonia concentration increases, the solubility of hexamine in ammonia solution will decrease. Hexamine will be decomposed in presence of hydrochloric acid, sulphuric acid, acetic acid and salicylic acid to form formaldehyde, ammonia, sodium carbonate, and methylamine. The higher H+
concentration is, the faster the decomposition will take place.
Section B. Hexamine Production Technology
Hexamine is prepared either by liquid phase process or by gas phase process. Both processes use formaldehyde and ammonia as raw materials. In liquid phase method, 37% aqueous formaldehyde solution is employed to react with ammonia gas. The formed hexamine solution is dehydrated through evaporation, centrifuged, and dried to receive solid product. As certain amount of steam is consumed in the vaporization, concentration and drying process, the energy consuming is high. This process is superior in mature technology and simple operation. Also the size of obtained hexamine particle is big because of the use of evaporation port and relative long crystallization time. In gas phase method, formaldehyde gas directly converted from methanol is sent to the ammoniation reactor to react with ammonia gas in the saturated hexamine mother liquid to form hexamine product. The feature of this process is that formaldehyde latent heat and hexamine reaction heat is used to evaporate water under vacuum condition.Thus large amount of steam is saved and high purity product is received. 1. Liquid phase production process Liquid phase production process is that 37% aqueous formaldehyde solution reacts with ammonia gas to form hexamine and water in an exothermic way. The reaction equation is as following: 6CH2 O(liquid) + 4NH3 gasCH2 6 N 4 + 6H2O + 81Kcal/mol The process includes several parts, i.e., condensation reaction between formaldehyde and ammonia, hexamine evaporation, concentration, centrifuging and drying. Details are given below : Ammonia gas is filtered, mixed with formaldehyde solution in a certain proportion (ammonia excesses 0.8-1.2%, and then sent to the reactor to yield 24-27% hexamine solution. The reaction heat is transferred out by circulation cooler to keep reaction temperature at 60-65. Hexamine solution is pumped to film
evaporator to be concentrated to 60-65% under vacuum. The generated moisture is condensed . 60-65% hexamine solution is fed to the evaporation boiler for further dehydration and concentration under vacuum. When crystal grains are formed, the mother liquid is sent to the centrifuge for separation. The obtain solid is supplied to the gas flow dryer. After cyclone separation, hexamine is received as final product. In this liquid phase method, evaporation is a continuous process while evaporation port operation is in a batch form , which entails high labor intensity and more waste water. Besides, the use of formaldehyde solution and the batch operation of concentration result in impurities and side reaction. As both of them have negative influence on the product purity, mother liquid filtering device must be set in the process line. 2. Gas phase method production process Gas phase production process is that formaldehyde and ammonia gas go through condensation reaction in alkaline solution. The reaction equation is given below 6CH2 O(gas) + 4NH3 gasCH2 6 N4 + 6H2O +745.29 103Kcal/mol In order to promote the reaction to proceed towards hexamine yielding direction and avoid side reactions(such as the forming of trimethylamine ), which may affect product quality and increase consumption, reaction temperature must be properly controlled and excess ammonia must be supplied. The production details are as follows:
PFD of Gas Phase Method for Hexamine
High temperatured-formaldehyde gas from oxidator is directly pumped to the amination reactor; ammonia gas after filtration and measuring is also sent to the ammoniation reactor. The two gases are bubbled in hexamine mother liquid to react with each other to form hexamine. Formaldehyde latent heat and hexamine reaction heat is used to evaporate water under vacuum. Reaction temperature is controlled at 75-80. The formed hexamine in the amination reactor is sent to receiving tank through sealing port. The mother liquid is returned to amination reactor, while concentrated liquid is supplied to the centrifuge to separate liquid phase. The obtained hexamine solid from the centrifuge is sent to the dryer for further dehydration before final product is received Excess ammonia, with the evaporated gas from reactor, is fed to the ammonia absorption tower for absorption. The unabsorbed gas is condensed and vacuumed to give the final off- gas , which enters into incinerator for burning after scrubbing. The diluted ammonia solution goes through recovery in stripper tower, and then is returned to ammoniation reactor; while the diluted methanol solution goes through recovery in recovering tower and then is returned to evaporator. Gas phase method allows continuous and automatic operation, short process line, less side reactions and high purified products. Also less environmental pollution and consumption is ensured because of ammonia and methanol recovery.
Section C. Material Consumption Quota
Materials Consumption Quota (Calculated based on per ton of 37% formalin) No. 1 2 3 4 5 6 Name Formaldehyde 37% Ammonia100% Circulation Cooling Water Steam0.4mpa Electric Power Salt Free Water Unit t kg t t KW.h t Liquid Phase Process 3.5-3.6 540-550 300 4.0-5.0 200 2 Gas Phase Process 3.5 530 150 0.51.0 300 1-2
10000t/a Hexamine Plant
10000t/a Hexamine Plant
10000t/a Hexamine Plant
20000t/a Hexamine Plant
You might also like
- Formaldehyde Production From MethanolDocument2 pagesFormaldehyde Production From MethanolDhang Siva100% (1)
- H ExamineDocument86 pagesH Examinemanoranjan singhNo ratings yet
- Project Report On HexamineDocument64 pagesProject Report On Hexaminetarunbnsl57% (7)
- Para FormaldehydeDocument5 pagesPara FormaldehydeVinh Do Thanh100% (1)
- Fractional DistillationDocument15 pagesFractional DistillationAlyan Srijaya0% (1)
- Formaldehyde Production From MethanolDocument40 pagesFormaldehyde Production From MethanolGreg Voloshenko83% (24)
- Thionyl Chloride ReactionsDocument7 pagesThionyl Chloride ReactionsMaxim MaximovNo ratings yet
- The Reaction Between Formaldehyde and AmmoniaDocument6 pagesThe Reaction Between Formaldehyde and AmmoniaalchemistraNo ratings yet
- A Convenient Way To Synthesis of Analgesic TramadolDocument1 pageA Convenient Way To Synthesis of Analgesic TramadolFacundo BaróNo ratings yet
- Report 1 - Feasibility Study For Formalin ProductionDocument51 pagesReport 1 - Feasibility Study For Formalin ProductionGizem Arslan100% (2)
- Dehydrogenation of Isopropyl Alcohol To AcetoneDocument1 pageDehydrogenation of Isopropyl Alcohol To Acetoneameyakem100% (1)
- Background: 1. Bromine 2. Caustic Potash (KOH) 3. AcetamideDocument2 pagesBackground: 1. Bromine 2. Caustic Potash (KOH) 3. AcetamideJunaid ahmedNo ratings yet
- Acetic AnhydrideDocument60 pagesAcetic Anhydridecyper zoonNo ratings yet
- Methylamine BrochureDocument4 pagesMethylamine BrochureRaven1013100% (1)
- Purification of MethanolDocument2 pagesPurification of Methanolmasthan6yNo ratings yet
- Formaldehyde Production 1Document8 pagesFormaldehyde Production 1Raymond FengNo ratings yet
- Nitric Acid Design Project: Shivam PandyaDocument15 pagesNitric Acid Design Project: Shivam PandyaShivam PandyaNo ratings yet
- Methanol Synthesis 1 ScribdDocument12 pagesMethanol Synthesis 1 ScribdgilmooodNo ratings yet
- Manufacturing of PentaerythritolDocument65 pagesManufacturing of Pentaerythritolsidd2706100% (1)
- Production of Formalin From Methanol: BackgroundDocument8 pagesProduction of Formalin From Methanol: BackgroundDeri PermanaNo ratings yet
- Methanol plaNT HYSYSDocument1 pageMethanol plaNT HYSYSarufatoNo ratings yet
- Packed Columns 110-126Document24 pagesPacked Columns 110-126tanveerpuNo ratings yet
- Energy Balance On Distillation ColumnDocument4 pagesEnergy Balance On Distillation ColumnCecilia Tan67% (9)
- Process Design of Monoethanolamine ProductionDocument83 pagesProcess Design of Monoethanolamine ProductionArpit Patel100% (1)
- Industrial Synthesis of Formic AcidDocument15 pagesIndustrial Synthesis of Formic Acidworlds tourNo ratings yet
- Phenol PlantDocument33 pagesPhenol PlantrakeshNo ratings yet
- N-Methyl Aniline & AnthraquinoDocument17 pagesN-Methyl Aniline & AnthraquinoSaifuddin AzizNo ratings yet
- Acetaldehyde Cost 2520Estimation&EconomicsDocument8 pagesAcetaldehyde Cost 2520Estimation&Economicsapi-3714811100% (1)
- Production of Methyl Ethyl KetoneDocument19 pagesProduction of Methyl Ethyl KetoneAditya JoshiNo ratings yet
- Preparation of Evidence in Illicit Amphetamine Manufacturing ProsecutionsDocument5 pagesPreparation of Evidence in Illicit Amphetamine Manufacturing Prosecutionsgeovani2No ratings yet
- SOP Mannheim Oven ProcessDocument1 pageSOP Mannheim Oven ProcessJomed Barallas100% (2)
- Daddy SynthesusDocument11 pagesDaddy SynthesusBarta BenceNo ratings yet
- Synthesis & Mathematical Modeling of PET Via Direct EsterificationDocument10 pagesSynthesis & Mathematical Modeling of PET Via Direct EsterificationMARKASGEORGENo ratings yet
- Methanol Plant 380 TPD 2362Document14 pagesMethanol Plant 380 TPD 2362Nontokozo Duma100% (1)
- Acetic AnhydrideDocument117 pagesAcetic AnhydrideEr Bali Pandhare87% (15)
- Chemical Kinetics On Thermal Decompositions of CumeneDocument8 pagesChemical Kinetics On Thermal Decompositions of CumeneMario Alonso Velasquez FlorezNo ratings yet
- Cumene Properties UsesDocument4 pagesCumene Properties UsesC.Çağrı Yekeler50% (2)
- Nitro Benzene Preparation, Laboratory & Industrial, Uses and ApplicationsDocument11 pagesNitro Benzene Preparation, Laboratory & Industrial, Uses and Applicationsusman_uet0881% (16)
- Caustic Soda (NaOH)Document15 pagesCaustic Soda (NaOH)Syed Ashmal HashmiNo ratings yet
- Dimethyl Aniline PDFDocument68 pagesDimethyl Aniline PDFVirendra RathvaNo ratings yet
- Manufacture of Ethyl Acrylate From Glycerol (2012)Document452 pagesManufacture of Ethyl Acrylate From Glycerol (2012)monericp100% (6)
- Haber - Bosch ProcessDocument12 pagesHaber - Bosch Processapi-487208181No ratings yet
- Maleic Anhydride - Process DesignDocument45 pagesMaleic Anhydride - Process Designstavros7100% (4)
- Acetaldehyde Methods 2520of 2520 ProductionDocument6 pagesAcetaldehyde Methods 2520of 2520 Productionapi-3714811100% (3)
- StyreneDocument167 pagesStyreneAdika SaputraNo ratings yet
- Absorption of Formaldehyde in WaterDocument135 pagesAbsorption of Formaldehyde in WaterBer GuzNo ratings yet
- Amination by ReductionDocument43 pagesAmination by ReductionShreyashNo ratings yet
- Hysys Methanol ProductionDocument1 pageHysys Methanol ProductionNguyễn Tiến Dũng100% (1)
- Production of Formaldehyde Project ReportDocument21 pagesProduction of Formaldehyde Project ReportU-sef Waleed100% (1)
- Purification of Laboratory Chemicals: Part 1 Physical Techniques, Chemical Techniques, Organic ChemicalsFrom EverandPurification of Laboratory Chemicals: Part 1 Physical Techniques, Chemical Techniques, Organic ChemicalsRating: 5 out of 5 stars5/5 (1)
- Legal Chemistry: A Guide to the Detection of Poisons, Examination of Tea, Stains, Etc., as Applied to Chemical JurisprudenceFrom EverandLegal Chemistry: A Guide to the Detection of Poisons, Examination of Tea, Stains, Etc., as Applied to Chemical JurisprudenceNo ratings yet
- Newer Methods of Preparative Organic Chemistry V2From EverandNewer Methods of Preparative Organic Chemistry V2Wilhelm FoerstNo ratings yet
- Hexamine 1 PDFDocument4 pagesHexamine 1 PDFPradhita Ramdani HNo ratings yet
- Energy Efficient Hexamine Production ProDocument4 pagesEnergy Efficient Hexamine Production ProMuhammad AbdullahNo ratings yet
- Efficient Hexamine ProductionDocument4 pagesEfficient Hexamine ProductionYousuck Donny ChandraNo ratings yet
- Melamine ProcessDocument18 pagesMelamine Processlinda pierre100% (1)
- New Microsoft Word DocumentDocument6 pagesNew Microsoft Word DocumentPinak ChowdhuryNo ratings yet
- ProjectDocument10 pagesProjectMuhammad JafarNo ratings yet
- Urea ProjectDocument17 pagesUrea ProjectAbdo Shaaban100% (2)
- Proper Air-Fuel Ratios for Starting, Idling, Accelerating & MoreDocument9 pagesProper Air-Fuel Ratios for Starting, Idling, Accelerating & MoreVinh Do ThanhNo ratings yet
- Air-Fuel Ratio, Lambda and Engine Performance: AFR M MDocument12 pagesAir-Fuel Ratio, Lambda and Engine Performance: AFR M MVinh Do ThanhNo ratings yet
- Studies On Drying Kinetics of Solids in A Rotary DryerDocument6 pagesStudies On Drying Kinetics of Solids in A Rotary DryerVinh Do ThanhNo ratings yet
- NPK-15 8 15Document5 pagesNPK-15 8 15Vinh Do ThanhNo ratings yet
- Multi-Use Chair DesignDocument7 pagesMulti-Use Chair DesignVinh Do ThanhNo ratings yet
- Modeling and Simulation of A Co-Current Rotary Dryer Under Steady ConditionsDocument8 pagesModeling and Simulation of A Co-Current Rotary Dryer Under Steady ConditionsVinh Do ThanhNo ratings yet
- Modelling and Simulation of A Direct Contact Rotary DryerDocument16 pagesModelling and Simulation of A Direct Contact Rotary DryerVinh Do ThanhNo ratings yet
- Ansi B16-104Document1 pageAnsi B16-104Monica Suarez100% (1)
- Effects of Drying Parameters On Heat Transfer During DryingDocument13 pagesEffects of Drying Parameters On Heat Transfer During DryingVinh Do ThanhNo ratings yet
- Dryer CalculationsDocument4 pagesDryer CalculationsVinh Do Thanh0% (1)
- The Heart of Operations - World Cement - 02-2015Document4 pagesThe Heart of Operations - World Cement - 02-2015fetniNo ratings yet
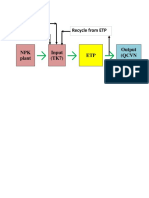
- Recycle From ETP Make Up H2O DAP, UreaDocument1 pageRecycle From ETP Make Up H2O DAP, UreaVinh Do ThanhNo ratings yet
- Equivalent Grades of Cast IronsDocument2 pagesEquivalent Grades of Cast IronsVinh Do ThanhNo ratings yet
- 4244 12672 1 PB PDFDocument15 pages4244 12672 1 PB PDFVinh Do ThanhNo ratings yet
- Aoac - Methods.1.1990. MoistureDocument2 pagesAoac - Methods.1.1990. MoistureVinh Do ThanhNo ratings yet
- DRS 279-2015 Organic Fertilizer - SpecificationDocument17 pagesDRS 279-2015 Organic Fertilizer - SpecificationVinh Do ThanhNo ratings yet
- PEP Report 267A: Ihs ChemicalDocument8 pagesPEP Report 267A: Ihs ChemicalVinh Do ThanhNo ratings yet
- 4244 12672 1 PB PDFDocument15 pages4244 12672 1 PB PDFVinh Do ThanhNo ratings yet
- Mau Giay Uy Quyen Bang Tieng AnhDocument3 pagesMau Giay Uy Quyen Bang Tieng AnhVinh Do ThanhNo ratings yet
- Natural Evaporation RateDocument16 pagesNatural Evaporation RateVinh Do ThanhNo ratings yet
- Metal Price IndexDocument1 pageMetal Price IndexVinh Do ThanhNo ratings yet
- Tinh Luong Nuoc Bay HoiDocument22 pagesTinh Luong Nuoc Bay HoiVinh Do ThanhNo ratings yet
- Review On Development of Polypropylene Manufacturing ProcessDocument11 pagesReview On Development of Polypropylene Manufacturing ProcessShweta Yadav100% (1)
- Estimating Evaporation From Water SurfacesDocument27 pagesEstimating Evaporation From Water SurfacesVinh Do ThanhNo ratings yet
- CRACKER A PC Based Simulator For Industr PDFDocument6 pagesCRACKER A PC Based Simulator For Industr PDFVinh Do ThanhNo ratings yet
- How To Calculate Heat Load - 5 StepsDocument1 pageHow To Calculate Heat Load - 5 StepsVinh Do ThanhNo ratings yet
- 1 0ProjectManagementProceduresDocument8 pages1 0ProjectManagementProceduresRamiesRahmanNo ratings yet
- Optimization of Wall Thickness For Minimum Heat LossesDocument9 pagesOptimization of Wall Thickness For Minimum Heat LossesVinh Do ThanhNo ratings yet
- How To Calculate Heat Load - 5 StepsDocument1 pageHow To Calculate Heat Load - 5 StepsVinh Do ThanhNo ratings yet
- Investigation of Sensible Heat Storage and Heat Insulation in The Exploitation of Concentrated Solar EnergyDocument5 pagesInvestigation of Sensible Heat Storage and Heat Insulation in The Exploitation of Concentrated Solar EnergyradanpetricaNo ratings yet
- What Is InterpolDocument5 pagesWhat Is InterpolJimmy Jr Comahig LapeNo ratings yet
- Mémoire ENSMDocument97 pagesMémoire ENSMAntoine Laurent100% (1)
- How COVID-19 Affects Corporate Financial Performance and Corporate Valuation in Bangladesh: An Empirical StudyDocument8 pagesHow COVID-19 Affects Corporate Financial Performance and Corporate Valuation in Bangladesh: An Empirical StudyInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- ATV600 - Communication - Parameters - EAV64332 - V1.3Document46 pagesATV600 - Communication - Parameters - EAV64332 - V1.3Sugeng WahyudiNo ratings yet
- Callon & Law (1997) - After The Individual in Society. Lessons On Colectivity From Science, Technology and SocietyDocument19 pagesCallon & Law (1997) - After The Individual in Society. Lessons On Colectivity From Science, Technology and Societysashadam815812No ratings yet
- Orgin of Life and Organic EvolutionDocument74 pagesOrgin of Life and Organic Evolutionasha.s.k100% (5)
- Architecture Vernacular TermsDocument3 pagesArchitecture Vernacular TermsJustine Marie RoperoNo ratings yet
- Bank 12Document19 pagesBank 12Shivangi GuptaNo ratings yet
- ELEC-E8714 Homework 3 - Life Cycle Assessment of LED Lamps - Manufacturing and UseDocument2 pagesELEC-E8714 Homework 3 - Life Cycle Assessment of LED Lamps - Manufacturing and UseŞamil NifteliyevNo ratings yet
- Build A Tunnel: What You NeedDocument2 pagesBuild A Tunnel: What You NeedManila Business ShopsNo ratings yet
- Acc121 Exam1 ProblemsDocument4 pagesAcc121 Exam1 ProblemsTia1977No ratings yet
- Hepatobiliary Surgery BlumgartDocument301 pagesHepatobiliary Surgery Blumgartaejazahsan100% (7)
- Si New Functions 3 1 eDocument152 pagesSi New Functions 3 1 ecmm5477No ratings yet
- Design Proposal For North Public & Suite Areas Decorative Lighting, Solaire Quezon CityDocument42 pagesDesign Proposal For North Public & Suite Areas Decorative Lighting, Solaire Quezon CityRichard Libunao BelduaNo ratings yet
- Angel FishDocument1 pageAngel FishWilla CrowellNo ratings yet
- Unit 6 ( CONSTRUCTION OF THE FLEXIBLE PAVEMENT )Document19 pagesUnit 6 ( CONSTRUCTION OF THE FLEXIBLE PAVEMENT )Zara Nabilah87% (15)
- Om 08.12.2022Document18 pagesOm 08.12.2022raviNo ratings yet
- Hevc StandardDocument11 pagesHevc Standardganesh gangatharanNo ratings yet
- Xbox - RGH E Ltu: Jogo 3.0 4.0 HD NºDocument11 pagesXbox - RGH E Ltu: Jogo 3.0 4.0 HD NºGabriel DinhaNo ratings yet
- Urinary: Rachel Neto, DVM, MS, DACVP May 28 2020Document15 pagesUrinary: Rachel Neto, DVM, MS, DACVP May 28 2020Rachel AutranNo ratings yet
- 04 Vendor Registration TrainingDocument16 pages04 Vendor Registration TrainingAhmad Ramin AbasyNo ratings yet
- GCAF Online Inspector Practice ExamDocument5 pagesGCAF Online Inspector Practice Examcamwills2100% (1)
- Gas Turbine MaintenanceDocument146 pagesGas Turbine MaintenanceMamoun1969100% (8)
- Service Manual: Applicable Models Model CodeDocument39 pagesService Manual: Applicable Models Model CodeAndres BicaNo ratings yet
- Lecture Notes On Revaluation and Impairment PDFDocument6 pagesLecture Notes On Revaluation and Impairment PDFjudel ArielNo ratings yet
- KD.7.1-WPS OfficeDocument9 pagesKD.7.1-WPS OfficePratista TyasNo ratings yet
- Department of Computer Applications Iii Semester Mc5304-Programming With JavaDocument32 pagesDepartment of Computer Applications Iii Semester Mc5304-Programming With JavaManibharathiNo ratings yet
- Effective Postoperative Pain Management StrategiesDocument10 pagesEffective Postoperative Pain Management StrategiesvenkayammaNo ratings yet
- GRADE-7 computer-MODULEDocument4 pagesGRADE-7 computer-MODULECzz ThhNo ratings yet
- tmp1AE2 TMPDocument8 pagestmp1AE2 TMPFrontiersNo ratings yet