Professional Documents
Culture Documents
Laboratory Quality Control Based on Risk Management Approach
Uploaded by
Mihai Ionita0 ratings0% found this document useful (0 votes)
571 views20 pagesCLSI document EP23 provides guidance for laboratories to develop a quality control plan (QCP) based on risk management. It emphasizes understanding all steps of the testing process to identify potential errors. The QCP incorporates controls to reduce risks and ensure quality. Laboratories should map their testing processes, identify risks, assess potential harms, and include appropriate controls in their customized QCP. Regular monitoring and review of the QCP helps improve efficiency and quality by preventing errors.
Original Description:
CLSI EP23-A
Laboratory Quality Control Based on Risk
Management; Approved Guideline
Original Title
Thank You! Clsi Ep23-A
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCLSI document EP23 provides guidance for laboratories to develop a quality control plan (QCP) based on risk management. It emphasizes understanding all steps of the testing process to identify potential errors. The QCP incorporates controls to reduce risks and ensure quality. Laboratories should map their testing processes, identify risks, assess potential harms, and include appropriate controls in their customized QCP. Regular monitoring and review of the QCP helps improve efficiency and quality by preventing errors.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
571 views20 pagesLaboratory Quality Control Based on Risk Management Approach
Uploaded by
Mihai IonitaCLSI document EP23 provides guidance for laboratories to develop a quality control plan (QCP) based on risk management. It emphasizes understanding all steps of the testing process to identify potential errors. The QCP incorporates controls to reduce risks and ensure quality. Laboratories should map their testing processes, identify risks, assess potential harms, and include appropriate controls in their customized QCP. Regular monitoring and review of the QCP helps improve efficiency and quality by preventing errors.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 20
CLSI EP23-A
Laboratory Quality Control Based on Risk
Management; Approved Guideline
Luann Ochs
CLIAC
February 14, 2012
EP23 The Right QC
EP23 IS NOT about reducing quality control
EP23 IS about understanding where errors can
occur, and putting the right controls in place to
reduce the risk of errors occurring
2
EP23 The Right QC
EP23 helps the lab director and the lab staff better
understand their entire testing process, from
collecting the sample to reporting the result
EP23 stresses the importance of, identifies, and
formalizes all of the other activities that labs do to
ensure quality results
EP23 expands the concept of what constitutes
quality control
3
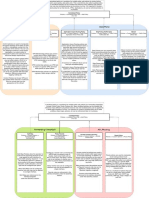
Overview of the EP23 Process
4
How does this work?
The lab director creates a Quality Control
Plan (QCP) based upon their testing
processes
Includes sample acquisition through result reporting
Includes analyzing of all process steps to see where
errors could occur, and what actions could prevent or
reduce the risk of that error occurring
Results in a plan that encompasses all of the identified
activities
Customized for each specific test, instrument, facility
5
The Benefits
CLSI document EP23 provides instruction
on developing an appropriate QCP that will:
Improve lab efficiency and quality- save time and
money through reducing errors
Appropriately use electronic and/or integrated QC
features
Incorporate all actions that ensure quality
Be truly customized for the specific laboratory and
facility situation
6
EP23 and The Quality Control Toolbox
Understand that each QC tool has its
strengths and weaknesses (there is no
perfect QC tool)
Implement a combination of tools in order to
properly control the test
EP23 explains the strengths and
weaknesses of the different types of QC
7
Introduces the Concept of Risk Assessment
Gather information from several sources:
Regulatory and accreditation requirements
Measuring system information
Health care and testing site settings
Medical requirements for the test results
Compile this information together by
creating a process map and a fishbone
diagram
8
Create a Process Map
9
Identify Key Process Steps
Once the process map is created, examine
it for places where errors could occur.
Five major areas:
Samples
Operator
Reagents
Laboratory environment
Measuring system
10
Group into a Fishbone Diagram
11
Perform the Risk Assessment
Identify the potential hazards and their
causes.
Assess each potential failure.
Where harm could occur, add an element in
the QCP that will reduce the severity of
harm, making residual risk acceptable.
For some types of failures, the manufacturers
information may already have a quality check in place.
12
Perform the Risk Assessment
Construct a table; see which types of errors
are detected and which ones are not.
If not detected, it must be included in the QCP.
For each possible hazard, assess the
severity of harm.
Do this for each hazard on five-point scales.
Use all of the information gathered in order to make
these assessments.
13
Perform the Risk Assessment
14
Definitions for categories are from ISO 14971
Assemble the Quality Control Plan
Use the chart developed earlier to add all of
the identified risks and their control
measures.
Construct the QCP.
Include each of the identified QCP Actions
in the QCP.
15
Monitor Quality Control Plan for Effectiveness
Verify that QCP put in place actually works
Continue to monitor errors and control
failures.
If an error occurs:
Take the appropriate corrective action.
Investigate the cause of the error.
Once the cause is understood, evaluate whether any
changes need to be made in the QCP.
16
Monitor Quality Control Plan for Effectiveness
Review any complaints that the laboratory
receives from health care providers.
These complaints may include pointing out another
source of QC failure that must be addressed.
For patient safety, review EP23 on a regular
basis to ensure an optimal QCP.
17
EP23 Summary
18
EP23 Companion Products
EP23 Implementation Workbook
EP23 Risk Assessment Worksheet
Template and Example
QCP Examples
Blood Glucose is in the EP23 document
PT/INR is in the workbook
Molecular test is coming soon
Blood gas test is in development
Webinars
For labs and for instrument/test manufacturers
19
Thank you!
20
You might also like
- ISO 15189 A Complete Guide - 2021 EditionFrom EverandISO 15189 A Complete Guide - 2021 EditionRating: 5 out of 5 stars5/5 (1)
- CLSI Guidelines Method EvaluationDocument53 pagesCLSI Guidelines Method Evaluationquanthuy100% (1)
- 5-Westgard QC StrategiesDocument36 pages5-Westgard QC StrategiesMarcos FernandezNo ratings yet
- CLSI Laboratory Documents Development and Control Approved Guideline NAT L COMM CLINICAL LAB STANDARDS 2006 PDFDocument100 pagesCLSI Laboratory Documents Development and Control Approved Guideline NAT L COMM CLINICAL LAB STANDARDS 2006 PDFErvin RodriguezNo ratings yet
- H4-A5 ClsiDocument60 pagesH4-A5 ClsiWilliams Alejandro Choroco Villegas100% (1)
- Free Download CLSI EP26 User Evaluation of Between Reagent Lot VariationDocument20 pagesFree Download CLSI EP26 User Evaluation of Between Reagent Lot VariationDrNikhil choudharyNo ratings yet
- Lot To Lot VariationDocument22 pagesLot To Lot VariationAwais Ahmad100% (1)
- Clsi HS2 ADocument92 pagesClsi HS2 AJuan Ignacio Cardenas RodriguezNo ratings yet
- Urinalysis 2021Document17 pagesUrinalysis 2021Tanu BhatiaNo ratings yet
- Clsi MM9 ADocument44 pagesClsi MM9 ACristian Gutiérrez VeraNo ratings yet
- Validation Vs VerificationDocument46 pagesValidation Vs VerificationMW100% (1)
- CAP Hematology 2013Document93 pagesCAP Hematology 2013Hassan GillNo ratings yet
- QC & QA in Hematology TestingDocument22 pagesQC & QA in Hematology TestingBenjamin Lopez Carreras100% (2)
- Sample: User Protocol For Evaluation of Qualitative Test Performance Approved Guideline-Second EditionDocument13 pagesSample: User Protocol For Evaluation of Qualitative Test Performance Approved Guideline-Second EditionjhoselineNo ratings yet
- Clsi Ep19 RDocument35 pagesClsi Ep19 RCristian Gutiérrez VeraNo ratings yet
- MANI Quality Control in Hematology AnalysersDocument65 pagesMANI Quality Control in Hematology Analyserscandiddreams100% (1)
- Blood Tube Collection GuideDocument365 pagesBlood Tube Collection GuideFeliciadlTNo ratings yet
- Clinical1 Quality Control - 10Document36 pagesClinical1 Quality Control - 10Khansa QureshiNo ratings yet
- IQCPDocument1 pageIQCPPrabhu Selvaraj100% (1)
- ISO 15189 ProceduresDocument63 pagesISO 15189 Proceduresaruba6250% (4)
- Laboratory Diagnosis of Urinary Tract Infections 2C Cumitech PDFDocument28 pagesLaboratory Diagnosis of Urinary Tract Infections 2C Cumitech PDFAlberto MontielNo ratings yet
- AACB 2013 Lab QCDocument9 pagesAACB 2013 Lab QCMW100% (1)
- Laboratory Quality ControlDocument63 pagesLaboratory Quality Control"DocAxi" Maximo B Axibal Jr MD FPSPNo ratings yet
- QC Rules Explained: Understanding Westgard Rules for Quality ControlDocument27 pagesQC Rules Explained: Understanding Westgard Rules for Quality Controlibrahim_barakatNo ratings yet
- ASCP Certification SOP PDFDocument2 pagesASCP Certification SOP PDFDeanne LambanNo ratings yet
- Evaluation of Precision Performance of Quantitative Measurement Methods Approved Guideline-Second EditionDocument52 pagesEvaluation of Precision Performance of Quantitative Measurement Methods Approved Guideline-Second EditionTrần Tiến Đạt100% (2)
- Clsi Catalog 2021 WebDocument34 pagesClsi Catalog 2021 WebPaul BandaNo ratings yet
- Guidance For Lab Quality Manuals v.3Document28 pagesGuidance For Lab Quality Manuals v.3Jason Roberts100% (1)
- Urine Culture Manual MT - SINAI PDFDocument15 pagesUrine Culture Manual MT - SINAI PDFAvi VermaNo ratings yet
- Procedures For The Collection of Diagnostic Blood Specimens by Venipuncture Approved Standard-Fifth EditionDocument52 pagesProcedures For The Collection of Diagnostic Blood Specimens by Venipuncture Approved Standard-Fifth EditionEiner JolberthNo ratings yet
- Measurement Procedure Comparison and Bias Estimation Using Patient Samples Approved Guideline-Third EditionDocument98 pagesMeasurement Procedure Comparison and Bias Estimation Using Patient Samples Approved Guideline-Third EditionTrần Tiến Đạt100% (10)
- HS10A2EDocument56 pagesHS10A2EВалерия БедоеваNo ratings yet
- Training and Competence Assessment Approved Guideline-Second EditionDocument60 pagesTraining and Competence Assessment Approved Guideline-Second EditionВалерия БедоеваNo ratings yet
- 3.6.6 SOP - Wound CultureDocument3 pages3.6.6 SOP - Wound CultureSemeeeJuniorNo ratings yet
- Guidelines Microbiology Lab ProceduresDocument61 pagesGuidelines Microbiology Lab ProceduresSadao MatsumotoNo ratings yet
- EP14A2Document44 pagesEP14A2Carlitasuasnavas100% (1)
- Method ValidationDocument34 pagesMethod ValidationNdra PompomorinNo ratings yet
- Statistical Approach in HematologyDocument33 pagesStatistical Approach in HematologycandiddreamsNo ratings yet
- Clsi La4 A4Document44 pagesClsi La4 A4Cristian Gutiérrez VeraNo ratings yet
- Cumitech Hemocultivos PDFDocument34 pagesCumitech Hemocultivos PDFAlberto MontielNo ratings yet
- TE, TEa, Six-SigmaDocument36 pagesTE, TEa, Six-SigmaDr. Pillala KrishnaveniNo ratings yet
- Glucose and CA19-9 MSA ResultsDocument5 pagesGlucose and CA19-9 MSA Resultszinniale_890% (1)
- QC-7 Hematology Westgard Rules in Good Performance LaboratoryDocument38 pagesQC-7 Hematology Westgard Rules in Good Performance LaboratoryWita100% (1)
- Interpretation of Westguard Rules in Clinical CemistryDocument26 pagesInterpretation of Westguard Rules in Clinical Cemistrylab_dep100% (4)
- Lecture Method ValidationDocument46 pagesLecture Method Validationsarah575No ratings yet
- Ep9 A2Document75 pagesEp9 A2lijiana100% (1)
- Evaluation of Precision Performance of Quantitative Measurement Methods Approved Guideline-Second EditionDocument56 pagesEvaluation of Precision Performance of Quantitative Measurement Methods Approved Guideline-Second EditionHassab Saeed100% (1)
- UnityDesktop BioRadDocument269 pagesUnityDesktop BioRadJose VarelaNo ratings yet
- Recommended TAE LimitsDocument4 pagesRecommended TAE LimitsMeidina Siti HanifahNo ratings yet
- Microbiology Best Laboratory PracticesDocument47 pagesMicrobiology Best Laboratory PracticesQAV_CRS100% (1)
- QCTrouble ShootingDocument59 pagesQCTrouble ShootingMustakim Duharing100% (3)
- Clsi-Catalog Web Links042414Document64 pagesClsi-Catalog Web Links042414PerezGut100% (1)
- Code of Ethics Guidance DocumentDocument30 pagesCode of Ethics Guidance DocumentASHVINBHAI VACHHANI100% (1)
- Iochemistry: DR Reema Bahri, MD Consultant - QA SRL Diagnostics PVT LTD, Mumbai 18 Dec' 2011Document51 pagesIochemistry: DR Reema Bahri, MD Consultant - QA SRL Diagnostics PVT LTD, Mumbai 18 Dec' 2011Is Arum100% (2)
- CLSI-Laboratory Documents - Development and Control - Approved Guideline-NAT'L COMM CLINICAL LAB STANDARDS (2006) PDFDocument100 pagesCLSI-Laboratory Documents - Development and Control - Approved Guideline-NAT'L COMM CLINICAL LAB STANDARDS (2006) PDFEman Bayoumi100% (4)
- Procedures For The Handling and Processing of Blood Specimens Approved Guideline - Third EditionDocument52 pagesProcedures For The Handling and Processing of Blood Specimens Approved Guideline - Third EditionВалерия Бедоева100% (1)
- Flow Cell Wash Kit Exp wsh004 WFC - 9120 - v1 - Revb - 08dec2020 MinionDocument9 pagesFlow Cell Wash Kit Exp wsh004 WFC - 9120 - v1 - Revb - 08dec2020 MinionErikk DangNo ratings yet
- Mls Study Guidelines AscpDocument5 pagesMls Study Guidelines AscpCielamae Orbeta100% (1)
- GMO-JRC Compendiu 2011Document259 pagesGMO-JRC Compendiu 2011Mihai IonitaNo ratings yet
- QT-NK 603Document3 pagesQT-NK 603Mihai IonitaNo ratings yet
- STEEL, P - The Nature of Procrastination - A Meta-Analytic and Theoretical Review of Quintessential Self-Regulatory FailureDocument30 pagesSTEEL, P - The Nature of Procrastination - A Meta-Analytic and Theoretical Review of Quintessential Self-Regulatory FailureRildo Polycarpo Oliveira75% (4)
- Report 26.05.2015 08.17.01Document6 pagesReport 26.05.2015 08.17.01Mihai IonitaNo ratings yet
- 24039Document130 pages24039Mihai IonitaNo ratings yet
- 10 Tips To Improve Your Pipetting TechniqueDocument1 page10 Tips To Improve Your Pipetting TechniqueMihai IonitaNo ratings yet
- fm3 6Document81 pagesfm3 6Mihai IonitaNo ratings yet
- Keeping A Good Laboratory Record Book: CompleteDocument4 pagesKeeping A Good Laboratory Record Book: CompleteMihai IonitaNo ratings yet
- Imagin I Cro Mozo MiDocument11 pagesImagin I Cro Mozo MiMihai IonitaNo ratings yet
- Microbial Endemism and BiogeographyDocument8 pagesMicrobial Endemism and BiogeographyMihai IonitaNo ratings yet
- Imagin I Cro Mozo MiDocument11 pagesImagin I Cro Mozo MiMihai IonitaNo ratings yet
- MRC CatalogueDocument105 pagesMRC CatalogueMihai IonitaNo ratings yet
- 2012 Update Networking - WS Colombia - QuerciDocument31 pages2012 Update Networking - WS Colombia - QuerciMihai IonitaNo ratings yet
- Practice UNIX Commands PDFDocument4 pagesPractice UNIX Commands PDFMihai IonitaNo ratings yet
- Stress-Responsive Gene ICE1 From Vitis Amurensis Increases ColdDocument6 pagesStress-Responsive Gene ICE1 From Vitis Amurensis Increases ColdMihai IonitaNo ratings yet
- Somaclonalvariancein DeschampsiaantarcticaDocument9 pagesSomaclonalvariancein DeschampsiaantarcticaMihai IonitaNo ratings yet
- Drum Filter EnglishDocument6 pagesDrum Filter EnglishMihai IonitaNo ratings yet
- How To Write A Standard Operating Procedure V 1Document3 pagesHow To Write A Standard Operating Procedure V 1Mihai IonitaNo ratings yet
- Protein Structure Determination: GoalDocument8 pagesProtein Structure Determination: GoalMihai IonitaNo ratings yet
- Process Level ChartDocument2 pagesProcess Level ChartzaidinNo ratings yet
- JETL industrial wastewater treatment reportDocument6 pagesJETL industrial wastewater treatment reportPremKumarNo ratings yet
- Mycom Nims ProptimaDocument4 pagesMycom Nims ProptimasamnemriNo ratings yet
- FADesignManual v2 14 SP PDFDocument88 pagesFADesignManual v2 14 SP PDFpandu lambangNo ratings yet
- Torque Specifications: Service Specifications - Ra60F Manual TransmissionDocument1 pageTorque Specifications: Service Specifications - Ra60F Manual TransmissionPedro Javier Castro SanchezNo ratings yet
- Solution 2 AntennaDocument7 pagesSolution 2 Antennaabdulwahab12100% (1)
- Canusa GTS - 3LPEDocument2 pagesCanusa GTS - 3LPEarifin rizalNo ratings yet
- Multi-disciplinary profile of IS/IT outsourcing researchDocument47 pagesMulti-disciplinary profile of IS/IT outsourcing researchIsabel MirandaNo ratings yet
- Sasirekha Computer ProjectDocument90 pagesSasirekha Computer ProjectAkurati RupendraNo ratings yet
- Implementing A Maintenance Strategic Plan Using TPM MethodologyDocument13 pagesImplementing A Maintenance Strategic Plan Using TPM MethodologyJeyson Lendro ParedesNo ratings yet
- Escorts F-15: Hydraulic Mobile Pick-n-Carry CraneDocument2 pagesEscorts F-15: Hydraulic Mobile Pick-n-Carry CraneChandra MouliNo ratings yet
- QlassicDocument31 pagesQlassicQila HusinNo ratings yet
- Wrf736sdam14 - Tech - Sheet - W10811276 - Rev - B Otro Modelo de La Nevera de Oliva OcoaDocument5 pagesWrf736sdam14 - Tech - Sheet - W10811276 - Rev - B Otro Modelo de La Nevera de Oliva OcoaEdison EspinalNo ratings yet
- Grove GMK 4080Document6 pagesGrove GMK 4080Deiver BarrazaNo ratings yet
- SYNOPSIS For Job PortalDocument6 pagesSYNOPSIS For Job PortalAkanksha Verma90% (10)
- A JIT Lot Splitting Model For Supply Chain Management Enhancing Buyer Supplier Linkage 2003 International Journal of Production EconomicsDocument10 pagesA JIT Lot Splitting Model For Supply Chain Management Enhancing Buyer Supplier Linkage 2003 International Journal of Production EconomicsDaniel Renaldo SimanjuntakNo ratings yet
- Instructions For Installation, Operating and Maintenance InstructionDocument30 pagesInstructions For Installation, Operating and Maintenance InstructionmilacronNo ratings yet
- Plasticizer From Vegetable Oil DerivativesDocument8 pagesPlasticizer From Vegetable Oil Derivativesilan chertokNo ratings yet
- Mechanical Testing of MaterialsDocument38 pagesMechanical Testing of MaterialsAanand Rishabh DagaNo ratings yet
- Plant Cost EstimationDocument49 pagesPlant Cost EstimationAlpianto100% (1)
- Preliminary Pin-out for 12 Cylinder Engine Control UnitDocument6 pagesPreliminary Pin-out for 12 Cylinder Engine Control UnitSUELENNo ratings yet
- fEA CourseDocument3 pagesfEA CourseAnant KumbhojkarNo ratings yet
- You Yangs RP Visitor GuideDocument2 pagesYou Yangs RP Visitor GuideSomaNo ratings yet
- Multiple-Choice QuestionsDocument8 pagesMultiple-Choice Questionsvijayganesh pinisettiNo ratings yet
- 07 AlarmManagement enDocument39 pages07 AlarmManagement enLuis RodriguezNo ratings yet
- Cutting A GemDocument18 pagesCutting A Gemmobsivac100% (1)
- Ieee 1346-1998Document45 pagesIeee 1346-1998pepitorodirguez100% (1)
- HW03 5ad S19 PDFDocument2 pagesHW03 5ad S19 PDFbobNo ratings yet
- Cisco SD-WAN Policy Architecture - Dana Yanch PDFDocument1 pageCisco SD-WAN Policy Architecture - Dana Yanch PDFEmilio PazNo ratings yet
- XXXXXXX XXXXXXX: Pour Exemple: Pour Exemple: ArteorDocument5 pagesXXXXXXX XXXXXXX: Pour Exemple: Pour Exemple: ArteorGilbert MartinezNo ratings yet