Professional Documents
Culture Documents
View Reviews2 Pankaj Jariwala
Uploaded by
kishorechandra0 ratings0% found this document useful (0 votes)
55 views13 pagesREVIEWS
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentREVIEWS
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
55 views13 pagesView Reviews2 Pankaj Jariwala
Uploaded by
kishorechandraREVIEWS
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 13
VIEW / REVIEWS
Management of Cardiogenic Shock Complicating Acute Myocardial Infarction.
Pankaj Jariwala, Pravir Lathi, G. Ramesh, K. Sarat Chandra
INTRODUCTION
Cardiogenic shock is an important cause of
in-hospital mortality after AMI. Though
Cardiogenic shock commonly occurs
following AMI or ischemia, there are
multiple other causes producing this final
common pathway leading to death.
Cardiogenic shock may present on
admission or may develop after admission.
The timing of presentation depends upon
the pathophysiology behind the disease
involved.
1
Cardiogenic shock occurs approximately
in 5-8 % of patients admitted with
diagnosis of AMI. The incidence of
cardiogenic shock has not decreased over
period of time despite decrease in the
mortality after AMI with increasing use of
early revascularization. Several trials in
the recent past have shown that early
revascularization strategies are useful and
improve 6 and 12 month mortality among
those who survived.
2
INCIDENCE
The incidence of CS remained
approximately 7-8% between 1975 and
1988 in a community in Worcester,
Massachusetts, USA, where 14% of
patients received thrombolytics
3
. The
incidence of CS in the Global Utilization
of Streptokinase and TPA for Occluded
Coronary Arteries (Gusto 1) trial, in which
all patients received thrombolysis was 7.3
%
4
.
The extent of myocardial salvage from
reperfusion treatment decreases
exponentially with time to re-establishing
coronary flow. Unfortunately, there has
been little progress in reducing time to
hospital presentation over the past decade
5
and this perhaps accounts for the stagnant
incidence of cardiogenic shock in
community studies (7.1%).
6
Cardiogenic
shock also complicates non-ST elevation
acute coronary syndromes. The incidence
of shock in the PURSUIT trial was 2.9%
(which excluded ST-elevation MI)
7
similar
to the 2.5% incidence reported in the non-
ST elevation arm of the GUSTO II-B trial
(199495).
8
CS continues to complicate approximately
5% to 8% of STEMI and 2.5% of non-
STEMI cases
7,9,10
.
PROGNOSIS
The 7.2% of patients developing shock in
the GUSTO-I trial accounted for 58% of
the overall deaths at 30 days.
11
Similarly,
the 30 day death rates with non-ST
elevation MI cardiogenic shock in the
PURSUIT and GUSTO-II b databases
were 66% and 73%, respectively. Even
with early revascularization, almost 50%
die at 30 days.
The historic control mortality for CS
complicating AMI is 80%
12,13
. From 1975
to 1988, which antedates the widespread
use of reperfusion therapy, mortality rate
has been consistently high at 78%
3
.
PATHOPHYSIOLOGY
Cardiogenic shock complicating acute MI
is caused by predominant left ventricular
failure in 80% of cases
14,15
. In the
remaining 20%, shock results from right
ventricular infarction or mechanical
complications of MI, including ventricular
septal rupture, acute mitral regurgitation,
and free wall rupture. In patients who have
predominantly left ventricular failure,
extensive necrosis is evident and involves
more than 40% of the left ventricle on
autopsy
16
.
1
In many patients, acute occlusion of
the left anterior descending artery is the
culprit lesion and, in two thirds of all
patients, multi-vessel disease is present
17
.
The precipitating event leading to CS may
not necessarily be a massive MI secondary
to a single arterial occlusion, but the result
of multiple small MIs with the final
myocardial insult leading to a sudden loss
of critical functioning myocardial
mass
17,18
.
Infarct expansion and re-infarction
have been identified as important risk
factors for development of shock in
STEMI and non-STEMI. In settings of low
coronary blood flow, thrombus may
propagate to involve side branches and
increase infarct size.
MI is a hyper-coagulable clinical
state, and re-occlusion of re-canalized
arteries leading to re-infarction can occur
in upto 20% of patients undergoing
thrombolytic therapy and 2% of patients
post-PCI. Patients who experience
reinfarction have significantly increased
chance of developing cardiogenic
shock
19,20
.
The progressively downward course
that describes the clinical deterioration of
patients who have cardiogenic shock is
shown in Fig.1
21,15
The leading event of
ischemia and infarction leads to systolic
and diastolic heart failure which involves
the entire circulatory system with
compensatory mechanisms setting in
which leads to classical paradigm of
circulatory shock. The systemic
inflammatory response syndrome involves
many inflammatory markers part of
neurohormonal and cytokine system in
perpetuation of shock.
22
This leads to
hypoperfusion of all organs, particularly
vital organs including heart, brain and
kidneys. The coronary perfusion pressure
decreases further and adds to the already
existing state.
The initial insult of ischemia leads
to stunned myocardium. Most of the
patients having multivessel disease already
had hibernating myocardium which also
contributes to the functional loss of
myocardium. The ability of the unaffected
myocardium to be recruited to preserve
left ventricular function is impaired.
23
To increase cardiac output, the heart
rate increases, thereby increasing
myocardial oxygen consumption directly
and affecting coronary perfusion. As heart
rate increases, the time spent in diastole
decreases. The time for tissue perfusion
decreases resulting in further ischemia of
the subendocardium. The wave front of
ischemia results in eventual global
dysfunction and the spiral deterioration
continues. The resulting stuttering
ischemia and infarction eventually results
in left ventricular failure and death.
23
CLINICAL RECOGNITION
Early recognition of left ventricular (LV)
failure and low output state, the preshock
phase, is crucial for the success of
treatment and outcome. The pre-shock
phase is not easily recognizable. Although
CS is routinely diagnosed when
hypotension develops, high systemic
vascular resistance can maintain
reasonable systemic arterial pressure even
when severe depression of stroke volume
and end organ hypoperfusion are present.
A useful clue is tachycardia, which is often
due to a low stroke volume. Confirmation
of the diagnosis is the most important step
in the management of these patients.
24
In
the SHOCK trial registry, 64% of patients
presented with hypotension, evidence of
ineffective cardiac output (resting
tachycardia, altered mental status, oliguria,
cool peripheries), and pulmonary
congestion.
25
A substantial minority (28%)
presented with evidence of hypoperfusion
in the absence of pulmonary congestion
the silent lung syndrome. These latter
patients have an equal distribution of
anterior (50%) and non-anterior index
infarctions (50%) with pulmonary
capillary wedge pressure in the range of
21.56.7 mm Hg.
2
DIAGNOSTIC CRITERIA OF
CARDIOGENIC SHOCK
Cardiogenic shock is clinically defined as
a state of end-organ dysfunction caused by
hypoperfusion secondary to low cardiac
output with associated hypotension,
classically defined as a systolic blood
pressure less than 90 mm Hg.
26
Hochman
et al proposed criteria for diagnosis of
cardiogenic shock as shown in box 1.
The presence of clinically cardiogenic
pulmonary edema itself is evidence of
increased left ventricular end diastolic
pressure.
26
CS should be diagnosed in all
patients exhibiting signs of inadequate
tissue perfusion irrespective of blood
pressure.
OTHER CAUSES OF CARDIOGENIC
SHOCK
There are several possible causes of
cardiogenic shock in the setting of MIleft
ventricular dysfunction, right ventricular
dysfunction, and mechanical
complications. Ventricular septal rupture,
contained free wall rupture, and papillary
muscle rupture. Mechanical complications
must be strongly suspected in patients with
CS complicating nonanterior MI,
particularly a first MI. Hemorrhage,
infection, and/or bowel ischemia may
contribute to shock in the setting of MI. As
with mechanical complications, a high
index of suspicion is required to make
these diagnoses in MI patients.
MANAGEMENT OF CARDIOGENIC
SHOCK FOLLOWING AMI
The treatment of cardiogenic shock
patients consists of medical therapy,
percutaneous revascularization procedures,
cardiac surgery, and the implantation of
devices.
Right Heart Catheterization
Right heart catheterization is often not
necessary for diagnosis and need only be
performed when there is continued doubt
or to guide management when shock does
not rapidly resolve. There is retrospective
data on increased mortality hazard
associated with this procedure
27
. On the
other hand, data from the Global Use of
Strategies to Open Occluded Coronary
Arteries (GUSTO-I) trial
28
and the Should
We Emergently Revascularize Occluded
Coronaries in Cardiogenic Shock ?
(SHOCK) registry
29
suggest that it is not
harmful, and possibly beneficial, in terms
of outcome. The current American and
European guidelines recommend right
heart catheterization for patients who have
ST-elevation acute coronary syndromes
(ACS) and who have progressive heart
failure, cardiogenic shock or mechanical
complications, despite the paucity of
evidence proving its efficacy
30,31
Echocardiography
Doppler echocardiography is of paramount
importance for hemodynamic assessment
and for the evaluation of cardiac function,
valvular status, and mechanical
complications of ACS.
32
Perhaps this
noninvasive, readily available method has
supplanted right heart catheterization use
in most situations.
Echocardiography in cardiogenic shock
can help to
Evaluate left ventricular function
and myocardium at risk
Evaluate remote myocardial
segments
Screen for ventricular septal
rupture
Screen for severe mitral
regurgitation and proceed to
transoesophageal echocardiography
as needed
Look for tamponade/rupture
Assess right ventricular function
TREATMENT
In the early 80s, individual or single
centre observational data on early
3
revascularization strategy benefiting
patients with cardiogenic shock after AMI
were published, but large scale trials were
needed to prove the point. Hence, the
National Heart, Lung, and Blood Institute
funded the SHOCK trial in the USA, while
the SMASH trial in Switzerland evaluated
the same issue.
33,34
While SMASH failed
to recruit an adequate number of patients,
SHOCK reported an increase in 30 day
survival from 46.7% to 56.0% by the
adoption of an early revascularisation
strategy, but this absolute 9% difference
did not reach significance (p =0.11). On
follow up, the survival difference in favour
of the early revascularisation strategy
became larger and significant at six
months (36.9% v 49.7%, p =0.027) and
one year (33.6% v 46.7%) for an absolute
reduction of 13.2% (95% CI 2.2% to
24.1%, p <0.03) Fig.2 The benefit of early
revascularisation was large for those <75
years at 30 days.
Kaplan-Meier curve showing 12 month
survival in the early revascularization and
initial medical stabilization arms of the
SHOCK trial.
82
General Care and Resuscitation
These include correction of hypovolemia,
hypoxemia, acidosis, Aspirin and
intravenous Heparin. The most important
step is maintenance of mean arterial
pressure to prevent further damage to the
vital organs and halt the perpetuation of
circulatory shock. A mean arterial pressure
of 60 mm Hg is generally necessary for
tissue perfusion.
35
Intra-arterial
measurement of blood pressure is
advisable. Large-scale controlled studies
have not been performed to compare
different combinations of inotropes in
patients who have cardiogenic shock.
Inotropes
Dopamine and Dobutamine are the most
commonly used inotropic agents.
Dopamines myocardial inotropic and
chronotropic effects are mediated through
myocardial beta-1 adrenergic receptors,
and are most evident with doses above 5
micrograms/kg/minute. The theoretical
advantage of nephroprotective effect has
been not been proved in one of the recent
trials.
36
Dobutamine has both alpha and
beta-adrenergic agonistic activities. It
increases myocardial contractility through
beta adrenergic receptor stimulation, with
only a minute peripheral vasodilatory
effect. The drug is usually initiated at 2
micrograms/kg/minute and titrated
upwards according to the patients
response, usually to a maximum of 20
micrograms/kg/minute. Dobutamine is
often used together with low-dose
dopamine for the inotropic effect of the
former and suggested renal vasodilator
effect of the latter.
Norepinephrine is a potent alpha
adrenergic agonist with less marked beta-1
adrenergic agonistic properties and a short
half-life of 2 to 4 minutes.
Vasoconstriction is dose-related, and the
drug infusion is titrated upwards from an
initial low dose of 0.02 to 0.04
microgram/kg/minute to a dose that
maintains adequate systemic blood
pressure. Norepinephrine may be used in
conjunction with dopamine and
dobutamine; however, the cardiac effects
of these agents include worsening
ischemia and serious arrhythmias, and the
vasoconstriction induced by
norepinephrine can lead to end-organ
ischemia. Thus, one should avoid
prolonged triple or double inotropic
treatment.
Milrinone, a second-generation
phosphodiesterase inhibitor, can be used in
cardiogenic shock patients. Milrinone
prevents the breakdown of cyclic
adenosine monophosphate (cAMP), the
final common pathway for raising
intracellular calcium, thus increasing
inotropy. The significant vasodilatory
effect and relatively long plasma half-life
make Milrinone difficult to use as
4
monotherapy in patients who have
cardiogenic shock. Milrinone can be used
synergistically with other inotropic drugs
to enhance myocardial contractility
37
.
Levosimendan represents a new class of
positive inotropic agents. Levosimendan
causes conformational changes in cardiac
Troponin C during systole, leading to
sensitization of the contractile apparatus to
calcium ions
38,39
. In addition to its
positive inotropic action, Levosimendan
possesses vasodilating properties that
reduce cardiac preload and afterload.
Moreover, coronary blood flow is
enhanced and myocardial oxygen supply is
increased
40,41
. At present, however, use of
levosimendan is not recommended by the
manufacturer for the sole treatment of
cardiogenic shock, because of its
vasodilatory properties.
Based on effects of vasopressin in septic
shock
42
, J olly and colleagues
43
hypothesized that vasopressin would
increase mean arterial pressure without
altering pulmonary capillary wedge
pressure or cardiac index. In a cohort of
patients who developed refractory
cardiogenic shock after myocardial
infarction, vasopressin was associated with
increased mean arterial pressure and no
adverse effect on other hemodynamic
parameters
43
.
Randomized prospective trials are
warranted to confirm the utility and safety
of vasopressin in the setting of cardiogenic
shock after myocardial infarction.
Other measures including fluids and
diuretic therapy need intensive
measurement of pulmonary wedge
pressure with the help of right heart
catheterization and hourly measurement of
urine output. Arterial blood gas and
oxygen saturation should be monitored
with early institution of continuous
positive airway pressure or mechanical
ventilation as needed. The ECG should be
monitored continuously, and defibrillating
equipment, intravenous Amiodarone, and
Lidocaine should be readily available.
Thirty three per cent of patients in the
early revascularization arm of the SHOCK
trial had cardiopulmonary resuscitation,
sustained ventricular tachycardia or
ventricular fibrillation before
randomization.
33
Mechanical Circulatory Support
Mechanical circulatory devices play an
important role in providing temporary
support for patients in CS. Mechanical
devices are superior to pharmacological
therapy in CS in that perfusion is
supported without increasing myocardial
work and oxygen consumption.
Intra-Aortic Balloon Counterpulsation
Intraaortic balloon counter pulsation
(IABP) is the most commonly used
circulatory support device. A large,
nonrandomized cooperative study in the
prethrombolytic era showed that IABP
counterpulsation alone, without adjunctive
percutaneous coronary angioplasty
(PTCA) or coronary artery bypass graft
surgery (CABG) did not reduce mortality
rates, which remained disappointingly high
at 83%
44
. IABP therapy was, however,
highly effective in initial clinical
stabilization of patients, reversing end-
organ hypoperfusion in patients with AMI
and CS who proved refractory to
vasopressor therapy
44
. Recent reports have
suggested that IABP may be combined
with thrombolytic therapy to improve
reperfusion rates when CS is present, with
improvement of overall outcome
45-47
. In
one nonrandomized study, where more
than 40% of patients received thrombolytic
therapy, there was improved survival rate
of 46% in those who received IABP versus
38% in those who did not [P =0.001]
46
.
IABP is extremely useful especially in
patients in whom support of the circulation
is required for the performance of cardiac
catheterization and/or other interventional
revascularization procedures such as
PTCA or CABG. It is mandatory in
patients with mechanical complications of
AMI such as ventricular septal defect or
papillary muscle rupture as a temporary
support before surgical correction. Patients
5
treated with IABP in conjunction with
thrombolytic therapy had the lowest in-
hospital mortality rate (47%) in the
SHOCK registry.
48
The SHOCK registry
data suggest that initial stabilization of
cardiogenic shock patients using an IABP
may be associated with a 20% absolute
risk reduction in mortality.
48
Similarly,
retrospective analysis from the United
States National Registry of Myocardial
Infarction
5
has shown a 6% absolute
reduction in hospital mortality associated
with IABP use among cardiogenic shock
patients. IABP was recommended in the
SHOCK Trial protocol and used in 87% of
patients, for a median duration of
approximately 3 days. Overall, IABP
usage was not an independent predictor of
mortality at 12 months; however, in
patients who had systemic hypoperfusion
and whose hemodynamics and end-organ
perfusion were improved by IABP, the
mortality rates were lower at 12 months in
both the initial medical stabilization and
the ERV treatment groups
49
. According to
accumulated data and accepted guidelines,
all patients having cardiogenic shock
without contraindications should receive
IABP for at least 3 days or longer if
clinically indicated, for recovery from
myocardial stunning.
30,31
Other types of mechanical devices that are
currently used to provide circulatory
support: Ventricular assist device
50
,
percutaneous cardiopulmonary by-pass
with extracorporeal membrane
oxygenation (ECMO)
51
and hemopump
52
.
All have been successfully used in a
limited number of patients as temporary
support before interventions or as a bridge
to heart transplantation
53
. Left ventricular
assist devices were described in a few
retrospective single-center based studies as
a promising modality in the post-
myocardial infarction setting
54,55
.
Anecdotal case reports and case series
were published recently confirming the
emerging role of urgent cardiac
resynchronization therapy (CRT) in
inotropes-dependent patients
56-58
.
Thrombolysis
There are few randomized trials providing
data for assessment of the efficacy of
thrombolysis in patients presenting with
CS. Intracoronary streptokinase results in
low patency rates of only 43% for patients
in CS
59
. When reperfusion is established,
the mortality rate is significantly lower
than when intracoronary streptokinase fails
to establish reperfusion, 42% vs 84%,
respectively
60
. GISSI I is the only placebo-
controlled thrombolytic trial that assessed
mortality rates for patients who presented
in CS. Streptokinase alone without
adjunctive aspirin or anticoagulation
administered up to 12 hours after onset
(regardless of ECG findings) resulted in
the same mortality rate as the placebo
(70%).
61
The FTT group emphasized that
the possibility of saving seven lives in
every 100 patients treated represents a
large absolute benefit.
62
The more recent
GUSTO I and TIMI II trials, which
utilized more adjunctive anti-coagulation
reported mortality rates of 58% and 51% at
30 days and 6 weeks, respectively.
63,64
The
lack of apparent benefit of thrombolytic
agents in the treatment of cardiogenic
shock may be the result of reduced lysis of
thrombi in patients who have low
perfusion pressures
59
. Data from small
nonrandomized retrospective studies of
patients with shock complicating AMI
support the use of IABP in conjunction
with thrombolysis
45-47
.
Glycoprotein IIb/IIIa Receptor
Antagonists
The available data favor
administration of platelet IIb/IIIa
antagonists, particularly Abciximab, in
patients who have cardiogenic shock who
6
are undergoing percutaneous intervention,
unless the risk of bleeding is considered to
be excessive. In the recently published trial
65
, 40 patients who had ST-segment
elevation myocardial infarction and
cardiogenic shock were treated with
primary revascularization, abciximab,
heparin, and aspirin. The reported
mortality rate at 30 days was 42.5%.
Abciximab seemed to improve outcomes
of the younger population [<75 years)
versus elderly (>75 years), with mortality
rates of 24% versus 91%, respectively
(P<001)
65
. Also in those patients
presenting with NSTEMI with shock GP
IIb/IIIa inhibitors should be administered
before revascularization.
Other experimental agents like N-
Monomethyl-L-Arginine
66
, Tilarginine
Acetate Injection
67
Complement blocking
agents like Pexelizumab
68-70
, adenosine
71
,
Sodium/hydrogen-exchange
inhibitors.
72,73
, Antioxidants like
Recombinant human superoxide dismutase
(h-SOD)
74,75
, Glucose-insulin-potassium
infusion
76,77
did not demonstrate any
benefit in terms of reduction in serious
adverse outcomes.
Revascularization
Bypass surgery
Early studies of revascularization for
patients with cardiogenic shock focused on
surgical revascularization. Several small
surgical series reported relatively good
outcomes in patients who had cardiogenic
shock
78,79
. According to SHOCK trial data,
surgically revascularized patients benefited
mostly among 57 patients who underwent
bypass surgery, 30-m day mortality was
42.1%, versus 45.3% for those treated with
PCI. The trial was not designed to
compare percutaneous and surgical
revascularization strategies
26
. There are
several surgical techniques designed to
optimize outcomes for patients in
cardiogenic shock: the use of warm blood
cardioplegia enriched with glutamate and
aspartate, grafting of large areas of viable
myocardium first followed by treatment of
infarct-related artery last, and preference
of saphenous vein grafts for their high
initial flow rates and because they can be
rapidly harvested
14
. Pooled data from 25
non-randomized studies involving 391
patients in cardiogenic shock who
underwent CABG revealed a 35% in-
hospital mortality rate
80
, and is the lowest
mortality rate reported for CS in AMI. It is
possible that complete revascularization
achieved by CABG in this population is
the best therapy. Hence it is reasonable to
consider urgent CABG with IABP support
for CS patients if logistics permit in a
given setup.
Percutaneous Transluminal Coronary
Angioplasty
An important advance in the management
of CS complicating AMI is the use of
PTCA, which is a highly effective method
of restoring perfusion in the infarct related
artery. There are currently 22 published
studies with an aggregate of 646 patients
who underwent PTCA at some time during
their hospitalization for CS and AMI. The
pooled in-hospital mortality is 45%
80
,
which is lower than the historic control
mortality rate of 80%. When PTCA is
successful the mortality rates are
significantly lower than when it is not (33
vs 81%). In the GUSTO 1 trial, patients in
cardiogenic shock who were subjected to
angiography and PTCA performed within
the first 24 hours had a mortality rate of
38%, while the remaining patients had a
62% mortality rate at 30 days
81
. Two
prospective randomized trials evaluated
the role of revascularization among
patients who had cardiogenic shock. The
SMASH trial (Swiss Multicenter trial of
Angioplasty for Shock) involved 55
patients who had AMI complicated by
cardiogenic shock. They were randomized
to undergo revascularization (surgical or
percutaneous) versus medical therapy
alone
34
. SMASH was discontinued
7
prematurely due to low enrolment and
physician perception that revascularization
was beneficial. In this small study, a 9%
absolute reduction in 30-day mortality was
seen among patients treated with
revascularization (69% versus 78%). This,
however, did not achieve statistical
significance. The most important
randomized study in this context is the
SHOCK trial. In this study patients who
developed cardiogenic shock within the
first 36 hours of AMI (either ST-segment
elevation, or new left bundle-branch
block) were eligible for enrolment;
patients who had mechanical causes for
shock or predominantly right-ventricular
infarction were excluded
26,82
. In this study
an aggressive, invasive approach
(angioplasty or coronary artery bypass
surgery: emergency revascularization
[ERV], generally with IABP) was
compared with medical treatment, initial
medical stabilization (IMS), including
thrombolytic therapy and IABP.
Randomization was required within 12
hours of shock onset. Most patients
randomized to the ERV (n = 152)
underwent coronary angiography (97%),
and 87% underwent revascularization
(surgical or percutaneous). The primary
end point of the study was 30-day
mortality. In the ERV arm, the mortality
was 46.7%, as compared with 56.0% in the
conservative arm. The mortality difference
at 6 months achieved statistical
significance, and was lower in the ERV
group (50.3% versus 63.1%) . The survival
curves continued to diverge at 12 months
of follow-up
82
. In a prespecified subgroup
analysis, the benefits of ERV were seen
only in patients younger than 75 years of
age, in whom the 30-day mortality was
56.8% for the conservative arm versus
41.4% for the ERV arm, however, 30-day
survival among medically treated patients
older than 75 years tended to be better than
that of older patients in the ERV arm
(53.1% versus 75.0%).
14
The 13% absolute
reduction in mortality at 6 months
associated with the aggressive
revascularization strategy (50.3% versus
63.1%) was both statistically and clinically
significant. On the basis of the SHOCK
trial, the American College of
Cardiology/American Heart Association
(ACC/ AHA) guidelines for the treatment
of patients who have myocardial infarction
have included early revascularization for
shock as a Class I indication, particularly
for those less than 75 years of age
30
In the
Global Registry of Acute Coronary Events
(GRACE) study, percutaneous
revascularization with coronary stenting
was the most powerful predictor of
hospital survival among shock patients.
83
CONCLUSION
Cardiogenic shock continues to remain a
challenge for the cardiologist taking care
of patients of acute myocardial infarction.
Based on the data presented above, one
should adopt an aggressive approach with
emergency PCI with IABP for these
critically sick patients. Stenting supported
by IIb/IIIa blockers has to be part of the
interventional approach.
REFERENCES
1. J an Horak et al. New Strategies in the
Management of Cardiogenic Shock in Acute
Myocardial Infarction. Heart Views Vol. 1 No.
3 March/May 1999:70-6
2. Venu Menon, J udith S Hochman et al.
Management of Cardiogenic Shock
Complicating Acute Myocardial Infarction.
Heart 2002; 88: 531-537.
3. Goldberg RJ , Gore J M, Alpert J S, et al.
Cardiogenic shock after myocardial infarction:
Incidence and mortality from a community -
wide perspective, 1975 to 1988. N. Engl. J .
Med 1991;325:1117-1122.
4. The GUSTO investigators. An international
randomized trial comparing for thrombolytic
strategies for acute myocardial infarction. N.
Engl. J . Med.1993;329:673 -682.
5. Rogers WJ, Canto J G, Lambrew CT, et al.
Temporal trends in the treatment of 1.5 million
patients with myocardial infarction in the US
from 1990 through 1999. J Am Coll Cardiol
2000;36:205663.
6. Goldberg RJ , Samad NA, Yarzebski J , et al.
Temporal trends in cardiogenic shock
8
7. Hasdai D, Harrington RA, Hochman J S, et al.
Platelet glycoprotein IIb/IIIa blockade and
outcome of cardiogenic shock complicating
acute coronary syndromes without persistent
ST- segment elevation. J Am Coll Cardiol
2000;36:68592
8. Holmes DR Jr, Berger PB, Hochman J S, et al.
Cardiogenic shock in patients with acute
ischemic syndromes with and without ST-
segment elevation. Circulation
1999;100:206773.
9. Fox KA, Anderson FA J r, Dabbous OH, Steg
PG, Lopez-Sendon J , Van de Werf F, Budaj A,
Gurfinkel EP, Goodman SG, Brieger D.
Intervention in acute coronary syndromes: do
patients undergo intervention on the basis of
their risk characteristics? The Global Registry
of Acute Coronary Events (GRACE). Heart.
2007;93:177182.
10. Babaev A, Frederick PD, Pasta DJ , Every N,
Sichrovsky T, Hochman J S. Trends in
management and outcomes of patients with
acute myocardial infarction complicated by
cardiogenic shock. J AMA. 2005;294:448454.
11. Holmes DR J r, Bates ER, Kleiman NS, et al.
Contemporary reperfusion therapy for
cardiogenic shock: the GUSTO-I trial
experience. J Am Coll Cardiol 1995;26:668
74.
12. Rogers WJ , Botxlby LJ , Chandra NC, et al.
Treatment of myocardial infarction in the
United States (1990 to 1993). Observations
from the national registry of myocardial
infarction. Circulation 1994; 90:2103 -2114.
13. Scheidt S, Ascheim R, Killip T. Shock after
acute myocardial infarction: A clinical and
hemodynamic profile. J . Am. Coll. Cardiol.
1970; 26:556 -561.
14. Duvernoy CS, Bates ER. Management of
cardiogenic shock attributable to acute
myocardial infarction in the reperfusion era. J
Intensive Care Med 2005;20(4):18898.
15. Hollenberg SM, Kavinsky CJ , Parrillo J E.
Cardiogenic shock. Ann Intern Med 1999;131:
4759.
16. Page DL, Caulfield J B, Kastor J A, et al.
Myocardial changes associated with
cardiogenic shock. N Engl J Med
1971;285(3):1337.
17. Wong SC, Sanborn T, Sleeper LA, et al.
Angiographic findings and clinical correlates
in patients with cardiogenic shock
complicating acute myocardial infarction: a
report from the SHOCK Trial Registry. Should
we emergently revascularize occluded
coronaries for cardiogenic shocK? J Am Coll
Cardiol 2000;36(3 Suppl A):107783.
18. Alonso DR, Scheidt S, Post M, et al.
Pathophysiology of cardiogenic shock.
Quantification of myocardial necrosis, clinical,
pathologic and electrocardiographic
correlations. Circulation 1973;48(3):58896.
19. Hasdai D, Califf RM, Thompson TD, et al.
Predictors of cardiogenic shock after
thrombolytic therapy for acute myocardial
infarction. J Am Coll Cardiol 2000;35:13643.
20. Hochman J S, Boland J S, Sleeper LA, et al.
Current spectrum of cardiogenic shock and
effect of early revascularization of mortality.
Circulation 1995;91:87381.
21. Hochman J S. Cardiogenic shock complicating
acute myocardial infarction: expanding the
paradigm. Circulation. 2003;107:2998 3002.
22. Kohsaka S, Menon V, Lowe AM, et al.
Systemic inflammatory response syndrome
after acute myocardial infarction complicated
by cardiogenic shock. Arch Intern Med 2005;
165(14):164350.
23. Eve D. Aymong et al. Pathophysiology of
Cardiogenic Shock complicating Acute
Myocardial Infarction. Med Clin N Am 91
(2007); 701712.
24. Menon V, Slater J N, White HD, et al. Acute
myocardial infarction complicated by systemic
hypoperfusion without hypotension: report
from the SHOCK trial registry. Am J Med
2000; 108:37480.
25. Menon V, White H, Lejemtel T, et al. The
clinical profile of patients with suspected
cardiogenic shock due to predominant left
ventricular failure. A report from the SHOCK
trial registry. J Am Coll Cardiol 2000;
36:10716.
26. Hochman J S, Sleeper LA, Webb J G, et al.
Early revascularization in acute myocardial
infarction complicated by cardiogenic shock.
SHOCK Investigators. Should we emergently
revascularize occluded coronaries for
cardiogenic shock. N Engl J Med 1999;341(9):
62534.
27. Connors AFJ , Speroff T, Dawson NV, et al.
The effectiveness of right heart catheterization
in the initial care of critically ill patients.
SUPPORT investigators. J AMA
1996;276:88997.
28. Hasdai D, Holmes DR J r, Califf RM, et al.
Cardiogenic shock complicating acute
myocardial infarction: predictors of death. Am
Heart J 1999;138:2131.
29. Menon V, Sleeper LA, Fincke R, et al.
Outcomes with pulmonary artery
catheterization in cardiogenic shock [abstract].
J Am Coll Cardiol 1998;31(Suppl A):397A.
30. Antman EM, Anbe DT, Armstrong PW, et al.
ACC/AHA guidelines for the management of
patients with ST-elevation myocardial
infarction: executive summary: a report of the
9
31. Van de Werf F, Ardissino D, Betriu A, et al.
Management of acute myocardial infarction in
patients presenting with ST-segment elevation.
The Task Force on the Management of acute
Myocardial Infarction of the European Society
of Cardiology. Eur Heart J 2003; 24:2866.
32. Hasdai D, Lev EI, Behar S, et al. Acute
coronary syndromes in patients with pre-
existing moderate to severe valvular disease of
the heart: lessons from the Euro-Heart Survey
of Acute Coronary Syndromes. Eur Heart J
2003;24:6239.
33. Hochman J S, Sleeper LA, Godfrey E, et al.
Should we emergently revascularize occluded
coronaries for cardiogenic shock: an
international randomized trial of emergency
PTCA/CABG-trial design. Am Heart J 1999;
137:31321.
34. Urban P, Stauffer J C, Bleed D, et al. A
randomized evaluation of early
revascularization to treat shock complicating
acute myocardial infarction: the (Swiss)
multicenter trial of angioplasty for shock-
(S)MASH. Eur Heart J 1999; 20:10308.
35. LeDoux D, Astiz ME, Carpati CM, et al.
Effects of perfusion pressure on tissue
perfusion in septic shock. Crit Care Med
2000;28:272932.
36. Australian and New Zealand Intensive Care
Society (ANZICS) Clinical Trials Group.
Lowdose dopamine in patients with early renal
dysfunction: a placebo controlled randomised
trial. Lancet 2000;356:213943.
37. Colucci WS, Denniss AR, Leatherman GF, et
al. Intracoronary infusion of dobutamine to
patients with and without severe congestive
heart failure: dose-response relationships,
correlation with circulating catecholamines,
and effect of phosphodiesterase inhibition. J
Clin Invest 1988;81:110310.
38. Haikala H, Kaheinen P, Levijoki J , et al. The
role of cAMP- and cGMP-dependent protein
kinases in the cardiac actions of the new
calcium sensitizer, levosimendan. Cardiovasc
Res 1997;34:53646.
39. Figgitt DP, Gillies PS, Goa KL.
Levosimendan. Drugs 2001;61:61327.
40. Lilleberg J , Nieminen MS, Akkila J , et al.
Effects of a new calcium sensitizer,
levosimendan, on haemodynamics, coronary
blood flow and myocardial substrate utilization
early after coronary artery bypass grafting. Eur
Heart J 1998;19:6608.
41. Cleland JG, McGowan J . Levosimendan: a
new era for inodilator therapy for heart failure?
Curr Opin Cardiol 2002;17:25765.
42. Holmes CL, Walley KR, Chittock DR, et al.
The effects of vasopressin on hemodynamics
and renal function in severe septic shock: a
case series. Intensive Care Med 2001;
27:141621.
43. J olly S, Newton G, Horlock E, et al. Effect of
vasopressin on hemodynamics in patients with
refractory cardiogenic shock complicating
acute myocardial infarction. Am J Cardiol
2005; 96:161720.
44. Scheidt S, Wilaer G, Mueller H, et al.
Intraaortic balloon contrapulsation in
cardiogenic shock: Report of a cooperative
clinical trial. New Engl. J . Med.1973;288:979 -
984.
45. Ohman EM, George BS, White CS, et al for
the Randomized IABP Group. Use of aortic
contrapulsation to improve sustained coronary
artery patency during acute myocardial
infarction. Results of randomized trial.
Circulation 1994;99:792 -799.
46. Waksman R, Weis AT, Gotsman MS, Hasin Y.
Intraaortic balloon contrapulsation improves
survival in cardiogenic shock complicating
acute myocardial infarction. Eur. Heart J ,
1993;7:71 - 74.
47. Stomel RB, Razak M, Bates ER. Treatment
strategies foracute myocardial infarction
complicated by cardiogenic shock in a
community hospital. Chest 1994;105:997 -
1002.
48. Sanborn TA, Sleeper LA, Bates ER, et al.
Impact of thrombolysis, intra-aortic balloon
pump counterpulsation, and their combination
in cardiogenic shock complicating acute
myocardial infarction: a report from the
SHOCK trial registry. J Am Coll Cardiol
2000;36: 11239.
49. French J K, Miller D, Palmieri S, et al.
Cardiogenic shock treated with thrombolytic
therapy and intra-aortic balloon counter
pulsation: a report from the SHOCK trial
[abstract]. Circulation 1999;100:(Suppl I):I-
370.
50. Moritz A, Wolmer E. Circulatory support with
shock due to acute myocardial infarction. Ann
Thorac Surg.1993; 55:238 -244.
51. Overlie PA, Walter, PD, Hurd, HP, et al.
Emergency cardiopulmonary support with
circulatory support devices. Cardiology
1994;84:231 -237.
52. Smalling RW, Swceney M, Lachterman B, et
al. Transvalvular left ventricular assistance in
cardiogenic shock secondary to acute
myocardial infarction. J . Am. Col. Cardiol.
1994; 23:637 -644.
10
53. De Rose J J, Argenziano M, Sun BC, et al.
Implantable left ventricular assist devices: An
evolving long-term cardiac replacement
therapy. Ann. Surg.1997; 226:461-470.
54. Leshnower BG, Gleason TG, OHara ML, et
al. Safety and efficacy of left ventricular assist
device support in postmyocardial infarction
cardiogenic shock. Ann Thorac Surg 2006;
81(4):136570.
55. Tayara W, Starling RC, Yamani MH, et al.
Improved survival after acute myocardial
infarction complicated by cardiogenic shock
with circulatory support and transplantation:
comparing aggressive intervention with
conservative treatment. J Heart Lung
Transplant 2006; 25(5):5049.
56. J ames KB, Militello M, Gus B, et al.
Biventricular pacing for heart failure patients
on inotropic support, a review of 38
consecutive cases. Tex Heart Inst J
2006;33(1):1922.
57. Konstantino Y, Iakobishvili Z, Arad O, et al.
Urgent cardiac resynchronization therapy in
patients with decompensated chronic heart
failure receiving inotropic therapy. A case
series. Cardiology 2006;106(1):5962.
58. Cowburn PJ , Patel H, J olliffe RE, et al.
Cardiac resynchronization therapy: an option
for inotrope-supported patients with end-stage
heart failure? Eur J Heart Fail 2005;7(2): 215
7.
59. Prewitt RM, Gu S, Schick U, Ducas J .
Intraaortic balloon counter pulsation enhances
coronary thrombolysis induced by intravenous
administration of a thrombolytic agent. J. Am.
Coll. Cardiol 1994; 23:794-798.
60. Kennedy J W, Gensivic CG, Timmis GC,
Maynard C. Acute myocardial infarction
treated with intracoronary streptokinase: A
report of the Society of Cardiac Angiography.
Am. J . Cardiol. 1985;55:871-877.
61. Gruppo Italiano per lo studio della
streptokinassi nellInfarto Miocardico (GISSI).
Effectiveness of intravenous thrombolytic
treatment in acute myocardial infarction:
Lancet. 1986;I(8478):397-402.
62. Fibrinolytic Therapy Trialists (FTT)
Collaborative Group. Indications for
fibrinolytic therapy in suspected myocardial
infarction: Collaborative overview of early
mortality and major morbidity results from all
randomized trials of more than 1000 patients.
Lancet 1994;393(8893):311-322.
63. Anderson RD, Ohman EM, Holmes DS, et al.
Use of intraaortic balloon contrapulsation in
patients with cardiogenic shock: Observations
from the GUSTO 1 study. J Am. Col.
Cardiol.1997; 30:708-715.
64. Garrahy PJ , Hazlowa MJ , Forman S, Rogers
WJ : Has thrombolysis improved survival from
cardiogenic shock? Thrombolysis in
myocardial infarction(TIMI II) results.
Circulation. 1989; II623(abstr).
65. Zeymer U, Tebbe U, Weber M, et al, on behalf
of ALKK Study Group. Prospective evaluation
of early abciximab and primary percutaneous
intervention for patients with ST elevation
myocardial infarction complicated by
cardiogenic shock: results of the REO-SHOCK
trial. J Invasive Cardiol 2003;15(7):3859.
66. Cotter G, Kaluski E, Blatt A, et al.L-NMMA(a
nitric oxide synthase inhibitor) is effective in
the treatment of cardiogenic shock. Circulation
2000;101:135861.
67. Waknine Y. FDA approvals: orphan drugs
troxatyl, tilarginine acetate, aerosolized gene
therapy. Available at:
http://www.medscape.com/viewarticle/507286
. Accessed J une 24, 2005.
68. Fitch J C, Rollins S, Matis L, et al.
Pharmacology and biological efficacy of a
recombinant, humanized, single-chain
antibody C5 complement inhibitor in patients
undergoing coronary artery bypass graft
surgery with cardiopulmonary bypass.
Circulation 1999;100:2499506.
69. Granger CB, Mahaffey KW, Weaver WD, et
al. Pexelizumab. An anti-C5 complement
antibody, as adjunctive therapy to primary
percutaneous coronary intervention in acute
myocardial infarction: the Complement
inhibition in Myocardial infarction treated with
Angioplasty (COMMA) trial. Circulation
2003;108:118491.
70. Hughes S. ApexAMI pexelizumab trial to be
stopped early. Available at: www.theheart.org/
article/640883.do. Accessed February 3, 2006.
71. Mahaffey KW, Puma J A, Barbagelata A, et al.
Adenosine as an adjunct to thrombolytic
therapy for acute myocardial infarction: results
of a multicenter, randomized, placebo-
controlled trial: the Acute Myocardial
Infarction Study of Adenosine (AMISTAD)
trial. J Am Coll Cardiol 1999;34:171120.
72. Theroux P, Chaitman BR, Danchin N, et al.
Inhibition of the sodium-hydrogen exchanger
with cariporide to prevent myocardial
infarction in high-risk ischemic situations:
main results of the GUARDIAN trial.
Circulation 2000;102:30328.
73. Zeymer U, Suryapranata H, Monassier J P, et
al. The Na/H exchange inhibitor eniporide
as an adjunct to early reperfusion therapy for
acute myocardial infarction. Results of the
Evaluation of the Safety and Cardioprotective
Effects of Eniporide in Acute Myocardial
Infarction (ESCAMI) trial. J Am Coll Cardiol
2001;38:164450.
74. Flaherty J T, Pitt B, Gruber J W, et al.
Recombinant human superoxide dismutase (h-
11
79. Subramanian VA, Roberts AJ , Zema MJ , et al.
Cardiogenic shock following acute myocardial
infarction; late functional results after
emergency cardiac surgery.NYState J Med
1980; 80(6):94752.
SOD) fails to improve recovery of ventricular
function in patients undergoing coronary
angioplasty for AMI. Circulation
1994;89:1982 91.
75. Zhang Y, Patel AA, J uergens C, et al. N
Acetylcysteine improves myocardial salvage
after reperfusion therapy in humans [abstract].
Circulation 1999;100(Suppl I):I-371.
80. Moscucci M, Bates ER. Cardiogenic shock.
Cardiol. Clinic 1995;13:391-406.
81. Berger PB, Holmes DR, Stebbins AL, et al for
the GUSTO I investigators. Impact of an
aggressive invasive catherization and
revascularization strategy on mortality in
patients with cardiogenic shock in the Global
Utilization of Streptokinase and Tissue
Plasminogen Activator for Occluded Coronary
Arteries (GUSTO 1) trial. An observational
study. Circulation 1997;96:122-127.
76. Diaz R, Paolasso EA, Piegas LS, et al.
Metabolic modulation of acute myocardial
infarction. The ECLA(Estudios Cardiologicos
Latinoamerica) Collaborative Group.
Circulation 1998; 98:222734.
77. Mehta SR, Yusuf S, Diaz R, et al. CREATE-
ECLA Trial Group investigators. Effect of
glucose- insulin-potassium infusion on
mortality in patients with acute ST-segment
elevation myocardial infarction: the CREATE-
ECLA randomized controlled trial. J AMA
2005; 293(4):43746.
82. Hochman J S, Sleeper LA, White HD, et al.
One-year survival following early
revascularization for cardiogenic shock.
J AMA 2001;285:1902.
83. Dauerman HL, Goldberg RJ , White K, et al.
Revascularization, stenting, and outcomes of
patients with acute myocardial infarction
complicated by cardiogenic shock. Am J
Cardiol 2002;90:83842.
78. Dunkman WB, Leinbach RC, Buckley MJ , et
al. Clinical and hemodynamic results of intra-
aortic balloon pumping and surgery for
cardiogenic shock. Circulation 1972;46:465
77.
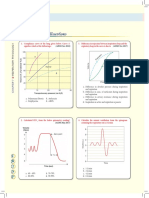
Fig 1
Fig 1. Current concept of CS pathophysiology. The classic description of CS pathogenesis is
shown in black. Myocardial injury causes systolic and diastolic dysfunction. A decrease in
CO leads to a decrease in systemic and coronary perfusion.This exacerbates ischemia and
causes cell death in the infarct border zone and the remote zone of myocardium. Inadequate
systemic perfusion triggers reflex vasoconstriction, which is usually insufficient. Systemic
inflammation may play a role in limiting the peripheral vascular compensatory response and
may contribute to myocardial dysfunction.Whether inflammation plays a causal role or is
only an epiphenomenon remains unclear. Revascularization leads to relief of ischemia. It has
not been possible to demonstrate an increase in CO or LVEF as the mechanism of benefit of
12
revascularization; however, revascularization does significantly increase the likelihood of
survival with good quality of life. IL-6 indicates interleukin-6; TNF-, tumor necrosis factor-;
and LVEDP, LV end-diastolic pressure. Adapted from Hochman 21 and Hollenberg et al 15
with permission of the publishers. Copyright 2003, the American Heart Association, and
copyright 1999, the American College of Physicians.
Fig 2: Kaplan-Meier curve showing 12 month survival in the early revascularization and
initial medical stabilization arms of the SHOCK trial.
82
Box 1
Hochman et al proposed criteria for diagnosis of cardiogenic shock as shown in box 1.
Address for correspondence: Dr. K. Sarat Chandra, Additional Professor, Department of
Cardiology Nizams Institute of Medical Sciences, Hyderabad 500 082
13
You might also like
- Neuroanatomy Quizbank QuestionsDocument18 pagesNeuroanatomy Quizbank Questionskishorechandra86% (29)
- Pathology Mock A.unlockedDocument75 pagesPathology Mock A.unlockedkishorechandraNo ratings yet
- Neet PSM - AspxDocument67 pagesNeet PSM - AspxkishorechandraNo ratings yet
- Viruses 02 00189Document24 pagesViruses 02 00189kishorechandraNo ratings yet
- Middle Ear Nerve Damage After Thyroid SurgeryDocument32 pagesMiddle Ear Nerve Damage After Thyroid Surgerykishorechandra75% (4)
- Adrplexus Chennai - Thrisur: Real Test Series - Subject Wise Test Series 2014Document1 pageAdrplexus Chennai - Thrisur: Real Test Series - Subject Wise Test Series 2014kishorechandraNo ratings yet
- S.No. Hospital Name Address State Specialty Acc. Upto Avl. SeatsDocument12 pagesS.No. Hospital Name Address State Specialty Acc. Upto Avl. SeatskishorechandraNo ratings yet
- Viruses 02 00189Document24 pagesViruses 02 00189kishorechandraNo ratings yet
- Reproduction Pathology ReviewDocument9 pagesReproduction Pathology ReviewkishorechandraNo ratings yet
- Department of Vaccines and BiologicalsDocument4 pagesDepartment of Vaccines and BiologicalsKishore Chandra KoradaNo ratings yet
- ACG Guidelines For Ulcerativecolitis PDFDocument15 pagesACG Guidelines For Ulcerativecolitis PDFkishorechandraNo ratings yet
- ECG PG - 23 LBBB - Details - (7 16.1 2011)Document7 pagesECG PG - 23 LBBB - Details - (7 16.1 2011)kishorechandraNo ratings yet
- 2004 20handbookDocument117 pages2004 20handbookkishorechandraNo ratings yet
- Maharastra Dental Paper - 2014Document16 pagesMaharastra Dental Paper - 2014kishorechandraNo ratings yet
- OrthopaedicsDocument25 pagesOrthopaedicskishorechandraNo ratings yet
- STI Operational GuidelinesDocument152 pagesSTI Operational GuidelineskishorechandraNo ratings yet
- 3 Pharmacology and Premedication PDFDocument10 pages3 Pharmacology and Premedication PDFMichael ThompsonNo ratings yet
- Dermatology - Appearances in Various DiseasesDocument12 pagesDermatology - Appearances in Various DiseaseskishorechandraNo ratings yet
- ANATOMY 1 NOTES: DELHI ACADEMY OF MEDICAL SCIENCESDocument140 pagesANATOMY 1 NOTES: DELHI ACADEMY OF MEDICAL SCIENCESAbhishek ChakravortyNo ratings yet
- Rheumatoid Arthritis Associated Scleritis OPHTHALMOLOGY MCQSDocument5 pagesRheumatoid Arthritis Associated Scleritis OPHTHALMOLOGY MCQSkishorechandraNo ratings yet
- ANATOMY Multiple Choice Questions 2012Document39 pagesANATOMY Multiple Choice Questions 2012kishorechandra100% (5)
- PGMEEnotes ENTnotes ENT Occasionally Asked TopicsDocument11 pagesPGMEEnotes ENTnotes ENT Occasionally Asked TopicskishorechandraNo ratings yet
- Thursday, April 11, 2013 5:41 PM: General Page 1Document3 pagesThursday, April 11, 2013 5:41 PM: General Page 1kishorechandraNo ratings yet
- Chapter 29 Capital Budgeting PDFDocument28 pagesChapter 29 Capital Budgeting PDFlalita pargaiNo ratings yet
- Viral InfectionsDocument17 pagesViral InfectionskishorechandraNo ratings yet
- UsmleDocument51 pagesUsmleapi-3822318100% (2)
- Immunology Aiims Type Exam 1 Fair Game Questions StudyBlueDocument14 pagesImmunology Aiims Type Exam 1 Fair Game Questions StudyBluekishorechandraNo ratings yet
- Typical RibDocument11 pagesTypical RibkishorechandraNo ratings yet
- 6th Central Pay Commission Salary CalculatorDocument15 pages6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Tugas GinjalDocument22 pagesTugas GinjalAnastasia MargaretNo ratings yet
- Nursing Care of a Child with Acute Myeloid LeukemiaDocument18 pagesNursing Care of a Child with Acute Myeloid LeukemiaStephanie Joy EscalaNo ratings yet
- Promoting Hygiene Through Complete BathingDocument12 pagesPromoting Hygiene Through Complete BathingErlangga PratamaNo ratings yet
- Pathologt of The HeartDocument40 pagesPathologt of The HeartJudithNo ratings yet
- Alternatives To Hysterectomy: Duncan Turner MD, MBBS, Facog, October 28th 1998 Santa Barbara, CaliforniaDocument39 pagesAlternatives To Hysterectomy: Duncan Turner MD, MBBS, Facog, October 28th 1998 Santa Barbara, Californiadekate_manojNo ratings yet
- Oral Ulcers: Acute and ChronicDocument39 pagesOral Ulcers: Acute and Chronicnour almarshadiNo ratings yet
- Relationship Between Body SystemsDocument2 pagesRelationship Between Body SystemsG8 Akshita BiswasNo ratings yet
- Anatomy & Physiology (Chapter 16 - Digestive System) Body SystemDocument29 pagesAnatomy & Physiology (Chapter 16 - Digestive System) Body SystemMarla TurquezaNo ratings yet
- Hematologic Reference Ranges: MSD Manual Veterinary ManualDocument3 pagesHematologic Reference Ranges: MSD Manual Veterinary ManualShiva KarthikNo ratings yet
- No. Kode Barang Nama Barang Exp. Date StockDocument12 pagesNo. Kode Barang Nama Barang Exp. Date StocknafilaNo ratings yet
- Psych Lab Finals (NCM 117)Document12 pagesPsych Lab Finals (NCM 117)Harvey T. Dato-onNo ratings yet
- A Project Report: Submitted By:-Preeti SinghDocument11 pagesA Project Report: Submitted By:-Preeti Singhkhushboo_khannaNo ratings yet
- Biology Chapter 40-1 Infectious DiseasesDocument23 pagesBiology Chapter 40-1 Infectious Diseasesapi-239353579No ratings yet
- CONTI Et Al. 2016 PDFDocument14 pagesCONTI Et Al. 2016 PDFJean SoaresNo ratings yet
- Wound HealingDocument23 pagesWound Healingkhadija HabibNo ratings yet
- Statement of Account For TuberculosisDocument1 pageStatement of Account For TuberculosisMHIEMHOINo ratings yet
- SBRT Pulmon Presentacion - TIPs and TricksDocument197 pagesSBRT Pulmon Presentacion - TIPs and TricksDavid GarciaNo ratings yet
- Ancient Greek and Greco-Roman Methods in Modern Surgical Treatment of CancerDocument3 pagesAncient Greek and Greco-Roman Methods in Modern Surgical Treatment of CancerAlexia ToumpaNo ratings yet
- Respiratory Physiology MCQsDocument25 pagesRespiratory Physiology MCQssk100% (5)
- 2 Part+1+Tutorial+DetailsadcDocument4 pages2 Part+1+Tutorial+DetailsadcAnup Lal RajbahakNo ratings yet
- CHANGES After Death (Part Two) 2011 GC - 2Document18 pagesCHANGES After Death (Part Two) 2011 GC - 2HassanNo ratings yet
- @MedicalBooksStoreS 2017 Acute IschemicDocument273 pages@MedicalBooksStoreS 2017 Acute IschemicAditya Perdana Dharma Wiguna100% (1)
- Abdominal Sepsis.15Document8 pagesAbdominal Sepsis.15Dario Xavier Achachi MelendezNo ratings yet
- Neonatal Tetanus SurveillanceDocument14 pagesNeonatal Tetanus SurveillanceNadiah Husnul KhotimahNo ratings yet
- Evidence-Based Medicine: David L. SackettDocument3 pagesEvidence-Based Medicine: David L. SackettNicolas MarinNo ratings yet
- Chapter 36 Skin Integrity and Wound CareDocument7 pagesChapter 36 Skin Integrity and Wound CareKathleen FrugalidadNo ratings yet
- Efficacy of Topical Tacrolimus 0.05% Suspension in Steroid-Resistant and Steroid-Dependent Vernal KeratoconjunctivitisDocument11 pagesEfficacy of Topical Tacrolimus 0.05% Suspension in Steroid-Resistant and Steroid-Dependent Vernal KeratoconjunctivitiselhambabaieNo ratings yet
- Gram Positive Cocci (GPC) Gram Neg (Rods GNR) Anaerobes Atypicals Classification AntibioticDocument2 pagesGram Positive Cocci (GPC) Gram Neg (Rods GNR) Anaerobes Atypicals Classification AntibioticRami RaedNo ratings yet
- Slit Skin SmearDocument12 pagesSlit Skin SmearRam ManoharNo ratings yet
- MemonDocument5 pagesMemonvamsi NathNo ratings yet