Professional Documents
Culture Documents
Lab Report #1 Photoelectric Effect
Uploaded by
John FiveCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lab Report #1 Photoelectric Effect
Uploaded by
John FiveCopyright:
Available Formats
The Photoelectric Effect
By: Yusuf Waxali
Lab Station #2
Lab Partner: John
February 13, 2014
Experiment #1 Photoelectric Effect
February 13, 2014
Photoelectric Effect
Objective
In this lab, students will observe the line spectrum emitted by a mercury vapor lamp.
Next, students will determine the most intense wavelength shining through colored
filters. Finally, students will observe the photoelectric effect and record the stopping
voltage for the three colored filters. From these results, students will calculate
experimental values for , h, v0, and 0.
Yusuf Waxali Physics 244
Experiment #1 Photoelectric Effect
February 13, 2014
Introduction
Crucial to the study of modern physics is the concept of wave-particle duality, or
the idea that something such as light may exhibit the behavior of a wave while being
comprised of discrete particles. While light certainly does behave like a wave,
undergoing reflection, refraction, and diffraction just as any other wave would, certain
behaviors of light cannot be explained by wave properties alone.
For example, consider the photoelectric effect. When light shines upon a metal
surface, some electrons are freed, producing a potential difference and thus electricity.
This phenomenon cannot be explained by simple wave properties. Rather, particle
behavior is required to make sense of the fact that light with certain wavelengths,
regardless of intensity, can liberate electrons from certain metal surfaces. As Einstein
postulated, the existence of photons, or the particles that make up light, was
responsible for the instantaneous release of electrons from the target surface.
It is for this reason that the photoelectric effect is an essential part of
understanding modern physics, which places much importance on the properties and
behavior of light.
In The Photoelectric Effect Experiment, students will observe the colors on the
mercury spectrum as ultraviolet light shines through a grating and on to a nearby piece
of paper. After noting which colors are most visibly intense on the paper, students
repeat with orange, green and blue filters. The respective wavelengths for each color
Yusuf Waxali Physics 244
Experiment #1 Photoelectric Effect
February 13, 2014
light are provided. Students then collect data for voltage readings when light shines on
the phototube in series connection with a voltmeter and an ammeter. After measuring
the voltage of the circuit, which is tangible evidence for the photoelectric effect, students
calculate values for the work function of the cesium antimonide phototube, Plancks
constant, threshold frequency, and threshold wavelength.
By analyzing the numbers behind the photoelectric effect through calculation and
experiment, The Photoelectric Effect Experiment captures the importance of the
photoelectric effect and helps bring better understanding to the subject of Modern
Physics.
Yusuf Waxali Physics 244
Experiment #1 Photoelectric Effect
February 13, 2014
Results
Data
After observing the light from the Mercury vapor lamp as it passes through a
grating, the colors were observed and described based on their relative apparent
intensity. See Table 1.
Table 1: Intensity of Spectral Lines from Mercury Vapor Lamp. Shows the
observed and actual relative intensities of each color of light, as well as
the wavelength and eye sensitivity
Students then inserted orange, green, and blue filters and observed the colors
that were transmitted through each filter. Each color of light was categorized as
eliminated, transmitted poorly, or transmitted well. This process was repeated for each
filter placed over the lamp. See Table 2.
Table 2: Effect of Filters. Shows the quality of transmission for each color of light
through each of the three filters
Yusuf Waxali Physics 244
Experiment #1 Photoelectric Effect
February 13, 2014
After connecting the phototube, voltmeter, and ammeter in series,
students measured the stopping voltage required to eliminate all of the
current in the circuit. This process was conducted three times, one for
each of the three filters. The wavelength () of the line responsible for the
stopping voltage was found based off of conclusions drawn from Table 2,
and the frequency (v) was calculated by dividing the speed of light by the
wavelength. See Table 3.
Table 3: Recorded Stopping Voltages and Wave Properties. Shows the
experimental stopping voltages for each filter as well as the wavelength
and frequency for each of the filters
Calculations
Please see the attached notebook paper with handwritten calculations.
Results
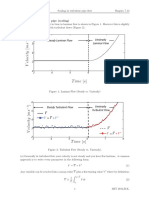
After getting three voltage readings for each filter, a total of nine extinction voltage
readings were plotted on a graph versus the frequency of each filter. The equation of
the line of best fit is shown on the right of the graph, along with the slope of the line of
best fit and the intercepts of the axes. See Figure 1.
Yusuf Waxali Physics 244
Experiment #1 Photoelectric Effect
February 13, 2014
Figure 1: Extinction Voltage vs. Frequency. Shows a scatter plot of all of the
experimental values for the extinction voltage. Includes equation,
slope, and intercept values for the line of best fit.
Finally, after collecting all of the data and plotting it, the intercepts and slope values
allow the student to calculate h, , v0, and o using the following equations:
1.
2.
For a table of the calculated values, see Table 4.
Yusuf Waxali Physics 244
Experiment #1 Photoelectric Effect
February 13, 2014
Table 4: Experimental Values. Shows calculated and published values for
Plancks constant, the work function of CsSb, and the threshold
frequency and wavelength for CsSb.
Conclusion
To summarize, students experimentally found stopping voltages for a mercury
lamp with colored filters on it and used this data to calculate experimental values for
Plancks constant, the work function of CsSb, and the threshold wavelengths and
frequencies of the phototube. The results for threshold frequency were acceptable,
having the same order of magnitude as the published value. The experimental value for
threshold frequency was 2.5E14 Hz. The work function was also acceptable, having the
same order of magnitude as the published work function value. The experimental value
for the work function was 2.78 eV. On the other hand, the experimental Plancks
constant was flawed, varying from the published value by 7 orders of magnitude. The
experimental value for Plancks constant was 1.78E-27 J*s.
One potential source of error was the use of the yellow filter rather than the green
filter. This could have affected the results since the wavelength and frequencies were
given for a green filter but the filter used was yellow. After omitting these data points
and using only the voltage readings from the blue and orange filters, the new
experimental value for h is 2.53E-34, which is much closer to the published value.
Yusuf Waxali Physics 244
You might also like
- The Photoelectric EffectDocument11 pagesThe Photoelectric EffectAlex Tarr100% (1)
- Photoelectric Effect Lab ReportDocument5 pagesPhotoelectric Effect Lab Reportsans_serif100% (2)
- 4c Lab9 Photoelectric EffectDocument4 pages4c Lab9 Photoelectric Effectk03mNo ratings yet
- (MIT) The Photoelectric EffectDocument4 pages(MIT) The Photoelectric EffectTVFionnaXQNo ratings yet
- Chapter 3 Solutions Modern Physics 4th EditionDocument24 pagesChapter 3 Solutions Modern Physics 4th EditionPaulo FontesNo ratings yet
- 03 Modern Photoelectric Effect LabDocument3 pages03 Modern Photoelectric Effect LabJuan David ParraNo ratings yet
- Photoelectric EffectDocument6 pagesPhotoelectric Effectabc abc100% (1)
- Photoelectric Effect Lab Report - KatzerDocument4 pagesPhotoelectric Effect Lab Report - Katzerapi-489811472No ratings yet
- Determination of Plancks Constant Using The Photoelectric EffectDocument5 pagesDetermination of Plancks Constant Using The Photoelectric EffectJp SwanepoelNo ratings yet
- Photoelectric Effect Lecture NotesDocument33 pagesPhotoelectric Effect Lecture NotesSebastian Quiceno100% (1)
- Atomic PhysicsDocument18 pagesAtomic PhysicsAnonymous ffLrUFNo ratings yet
- Thermal Conductivity of A Bad ConductorDocument3 pagesThermal Conductivity of A Bad Conductorjoputa15No ratings yet
- Lab 1, Electric Fields and Equipotentials Data AnalysisDocument9 pagesLab 1, Electric Fields and Equipotentials Data AnalysisCuong Nguyen100% (1)
- Ferro Dia para Magnetism PDFDocument11 pagesFerro Dia para Magnetism PDFShanna-Kay Wood-Davidson100% (1)
- Photoelectric Effect PDFDocument7 pagesPhotoelectric Effect PDFVenu GopalNo ratings yet
- Modern PhysicsDocument34 pagesModern PhysicsAditya BansalNo ratings yet
- (#3) Direct, Indirect, Ek Diagram, Effective Mass PDFDocument6 pages(#3) Direct, Indirect, Ek Diagram, Effective Mass PDFMas RoorNo ratings yet
- 8.work Function of MetalDocument3 pages8.work Function of MetalRavi Kanth M NNo ratings yet
- Physics Lab Report AnalysisDocument76 pagesPhysics Lab Report AnalysisWaseem Akram50% (2)
- Kinetic Theory of GasesDocument8 pagesKinetic Theory of GasesGupta GuptaNo ratings yet
- Concepts of Modern Physics SolutionsDocument166 pagesConcepts of Modern Physics SolutionsKonrad Nied33% (3)
- Photoelectric Effect Lecture NotesDocument37 pagesPhotoelectric Effect Lecture NotesFaizan Khan100% (2)
- New Modern PhysicsDocument3 pagesNew Modern PhysicsshabbirtechnicalNo ratings yet
- Practica 4. Efecto FotoelectricoDocument2 pagesPractica 4. Efecto FotoelectricoAdrian Herrera PinoNo ratings yet
- Thermal Conductivity Measurement For Poor Conductor - Lee's DiscDocument17 pagesThermal Conductivity Measurement For Poor Conductor - Lee's Discibnu_iman810% (2)
- Franck Hertz ExperimentDocument15 pagesFranck Hertz Experimentlalal345No ratings yet
- How Wire Resistance Varies with TemperatureDocument2 pagesHow Wire Resistance Varies with TemperatureAdán Shennan Farpón33% (6)
- Measure Unknown Resistance Using Wheatstone BridgeDocument4 pagesMeasure Unknown Resistance Using Wheatstone BridgeJohn Wang100% (2)
- Dielectric ConstantDocument106 pagesDielectric Constantعلى حسنNo ratings yet
- InterferenceDocument19 pagesInterferenceMuhammad AliNo ratings yet
- Modern Physics NotesDocument157 pagesModern Physics NotesGregory HillhouseNo ratings yet
- Geometric Optics Multiple Choice QuestionsDocument14 pagesGeometric Optics Multiple Choice QuestionsqvrlenarasegtNo ratings yet
- The Schrödinger Equation in One DimensionDocument23 pagesThe Schrödinger Equation in One DimensionjunaidNo ratings yet
- 4 - Dielectric Constant of Different MaterialsDocument6 pages4 - Dielectric Constant of Different MaterialsPAULO CESAR CARHUANCHO VERA0% (1)
- OL Radioactivity and Nuclear PhysicsDocument24 pagesOL Radioactivity and Nuclear Physicssaifaly shaheenNo ratings yet
- 4 - B - Sc. I I JOURNELDocument80 pages4 - B - Sc. I I JOURNELasif_zehravi804883% (6)
- Manual of Modern Physics ExperimentDocument107 pagesManual of Modern Physics ExperimentRishabh SahuNo ratings yet
- Motion of Charged ParticleDocument27 pagesMotion of Charged ParticleGurvir SinghNo ratings yet
- Applied Physics Lab ManualDocument53 pagesApplied Physics Lab Manualarehanliaqat8125100% (3)
- Wave-Particle Duality EvidenceDocument24 pagesWave-Particle Duality EvidenceIsrael PopeNo ratings yet
- Coupled Pendulum-Lab ReportDocument6 pagesCoupled Pendulum-Lab ReportAhh Doh100% (1)
- 13 - ElectrostaticsDocument8 pages13 - Electrostaticsp_k_soni_iit_physics78% (9)
- Different Atomic Models SeminarDocument24 pagesDifferent Atomic Models SeminarReddyvari VenugopalNo ratings yet
- Lec 1 Solid State PhysicsDocument37 pagesLec 1 Solid State PhysicsAnkita RajputNo ratings yet
- A Physics PDFDocument123 pagesA Physics PDFArsalan UddinNo ratings yet
- Prism SpectrometerDocument8 pagesPrism SpectrometerAnuruddha Dilshan RathnayakeNo ratings yet
- Faraday's Law of Induction-1Document7 pagesFaraday's Law of Induction-1nandhakumarmeNo ratings yet
- Quantum Nature of Light ExplainedDocument6 pagesQuantum Nature of Light ExplainedddsddsddsNo ratings yet
- Conceptual Survey Electricity and MagnetismDocument12 pagesConceptual Survey Electricity and MagnetismsuhartojagoNo ratings yet
- CBSE XII Physics Board Paper SolutionDocument28 pagesCBSE XII Physics Board Paper SolutionTera BaapNo ratings yet
- Atomic PhysicsDocument55 pagesAtomic PhysicsPristiadi Utomo100% (2)
- Gamma Ray Spectroscopy ExperimentDocument15 pagesGamma Ray Spectroscopy ExperimentLizelleNiit100% (1)
- Physics 1922 – 1941: Including Presentation Speeches and Laureates' BiographiesFrom EverandPhysics 1922 – 1941: Including Presentation Speeches and Laureates' BiographiesNo ratings yet
- Lab Report #3 Emission Spectrum of HydrogenDocument7 pagesLab Report #3 Emission Spectrum of HydrogenJohn FiveNo ratings yet
- PHYS 102 REPORT 1Document2 pagesPHYS 102 REPORT 1mavrickgsniusNo ratings yet
- Exercise Paper On Photoelectric EffectDocument4 pagesExercise Paper On Photoelectric EffectJohn Lorenzo100% (1)
- Creative Tech PaperDocument5 pagesCreative Tech PaperJesha LibreaNo ratings yet
- Lagrange Polynomial: y (X) A X X X X X X X X X X X XDocument8 pagesLagrange Polynomial: y (X) A X X X X X X X X X X X XJohn FiveNo ratings yet
- CH 2: Fundamentals of Kinematics: - Topic OutlineDocument36 pagesCH 2: Fundamentals of Kinematics: - Topic OutlineJohn FiveNo ratings yet
- CH 4 Deflection and StiffnessDocument26 pagesCH 4 Deflection and StiffnessJohn FiveNo ratings yet
- Ex Mental Bran Research: Spatial Control of Arm MovementsDocument5 pagesEx Mental Bran Research: Spatial Control of Arm MovementsJohn FiveNo ratings yet
- Acceleration Graphical NotesDocument3 pagesAcceleration Graphical NotesJohn FiveNo ratings yet
- 01 Bragg ScatteringDocument8 pages01 Bragg ScatteringJohn FiveNo ratings yet
- Geiger CountsDocument1 pageGeiger CountsJohn FiveNo ratings yet
- Lab Report #3 Emission Spectrum of HydrogenDocument7 pagesLab Report #3 Emission Spectrum of HydrogenJohn FiveNo ratings yet
- Lab Report #2 Bragg ScatteringDocument10 pagesLab Report #2 Bragg ScatteringJohn FiveNo ratings yet
- The Effect of Carbon Content On The C A Ratio of As-Quenched Martensite in Fe-C AlloysDocument6 pagesThe Effect of Carbon Content On The C A Ratio of As-Quenched Martensite in Fe-C AlloysAlexis Guzmán MéndezNo ratings yet
- Datasheet Din 931Document6 pagesDatasheet Din 931Jelena DNo ratings yet
- Micro Interferometers for Biowarfare DetectionDocument17 pagesMicro Interferometers for Biowarfare DetectionKushal ChoudharyNo ratings yet
- Lecture 4: Plasticity: 4.1: Stiffness-Limited DesignDocument4 pagesLecture 4: Plasticity: 4.1: Stiffness-Limited DesignThomas Van KuikNo ratings yet
- Assign4 RANSDocument2 pagesAssign4 RANSankitsaneetNo ratings yet
- PRIPLAST Polyester Polyols Derived From Dimerized Fatty AcidsDocument30 pagesPRIPLAST Polyester Polyols Derived From Dimerized Fatty AcidsA MahmoodNo ratings yet
- Aeroshell Fluid 41: Main Applications Specifications, Approvals & RecommendationsDocument2 pagesAeroshell Fluid 41: Main Applications Specifications, Approvals & Recommendationsabdul rehmanNo ratings yet
- Corporation 100 CTC Drive Johnstown, PA 15904Document7 pagesCorporation 100 CTC Drive Johnstown, PA 15904Milan JoshiNo ratings yet
- Effect of Rotational Speed On The Performance of Unreinforced andDocument8 pagesEffect of Rotational Speed On The Performance of Unreinforced andmustafa taiforNo ratings yet
- CE 322 Mechanics of Deformable BodiesDocument41 pagesCE 322 Mechanics of Deformable BodiesKristine May MaturanNo ratings yet
- TR Rans FormerDocument401 pagesTR Rans FormerJohn Mark KiatNo ratings yet
- Air Circuit BreakerDocument18 pagesAir Circuit BreakerTalha SadiqNo ratings yet
- Design of Optimum HeatsinkDocument9 pagesDesign of Optimum Heatsinkbitconcepts9781No ratings yet
- Maxwell-Equations - Physics-PWRDocument18 pagesMaxwell-Equations - Physics-PWRRahul YadavNo ratings yet
- APA - TT-053 Shearwall Deflection FormulaDocument4 pagesAPA - TT-053 Shearwall Deflection FormulakmccrapmailNo ratings yet
- Thin Walled Pressure VesselDocument13 pagesThin Walled Pressure VesselaalijanaabNo ratings yet
- Astm D 6528 00Document9 pagesAstm D 6528 00Devendra ShrimalNo ratings yet
- Properties of Engineering Materials (Handouts)Document6 pagesProperties of Engineering Materials (Handouts)Vergel De TorresNo ratings yet
- AS 404 – STEEL AND TIMBER DESIGN CODEDocument4 pagesAS 404 – STEEL AND TIMBER DESIGN CODEJoshua VicenteNo ratings yet
- Material Selection Guide - Endura PlasticsDocument29 pagesMaterial Selection Guide - Endura PlasticsvadiNo ratings yet
- Dr. Sushma Gupta Professor Department of Electrical Engineering MANIT, BhopalDocument14 pagesDr. Sushma Gupta Professor Department of Electrical Engineering MANIT, BhopalVenomNo ratings yet
- 5 - FF - Fatigue MechanismDocument34 pages5 - FF - Fatigue MechanismmayankNo ratings yet
- ME-PhD Admission 2023Document1 pageME-PhD Admission 2023Dr-Bharath Vedashantha MurthyNo ratings yet
- Ansi A92.2.1969Document18 pagesAnsi A92.2.1969HermanRomanNo ratings yet
- A Literature Review On Friction Stir Welding of Similar and Dissimilar MaterialsDocument5 pagesA Literature Review On Friction Stir Welding of Similar and Dissimilar MaterialsKaushik SenguptaNo ratings yet
- Strength of Materials 2022 by S K Mondal 8 MB-edited PDFDocument338 pagesStrength of Materials 2022 by S K Mondal 8 MB-edited PDFRajesh sahaNo ratings yet
- T2voc ManualDocument168 pagesT2voc ManualThanh PhongNo ratings yet
- 7-Fluid SaturationDocument13 pages7-Fluid Saturationاحمد ابوبكر اشقيفهNo ratings yet
- 2 - 25F13 - Shap7.14 - SHAPIRODocument3 pages2 - 25F13 - Shap7.14 - SHAPIROBruno Henrique HoinschyNo ratings yet
- 9 10 enDocument2 pages9 10 enparth kananiNo ratings yet