Professional Documents
Culture Documents
A Chilly Fever: Clinical Problem-Solving
Uploaded by
Juan Carlos Carbajal SilvaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A Chilly Fever: Clinical Problem-Solving
Uploaded by
Juan Carlos Carbajal SilvaCopyright:
Available Formats
The
n e w e ng l a n d j o u r na l
of
m e dic i n e
clinical problem-solving
Caren G. Solomon, M.D., M.P.H., Editor
A Chilly Fever
Christin Price, M.D., Cameron Ashbaugh, M.D., Amy Leigh Miller, M.D., Ph.D.,
and Joseph Loscalzo, M.D., Ph.D.
In this Journal feature, information about a real patient is presented in stages (boldface type)
to an expert clinician, who responds to the information, sharing his or her reasoning with
the reader (regular type). The authors commentary follows.
A 30-year-old graduate student presented to the emergency department in March reporting fevers associated with shaking chills and severe headaches. He had been well
until 1 week before presentation, when he began to have daily fevers, with temperatures as high as 103.0F (39.4C) and with associated chills, nausea, and headaches.
The fevers were most notable in the evening; by morning, the patient was afebrile and
felt well enough to attend class. He had been taking acetaminophen, without symptom
relief.
From the Department of Medicine,
Brigham and Womens Hospital and Harvard Medical School, Boston. Address
reprint requests to Dr. Miller at 75 Francis
St., Boston, MA 02115, or at almiller@
partners.org.
N Engl J Med 2014;371:1833-7.
DOI: 10.1056/NEJMcps1313772
Copyright 2014 Massachusetts Medical Society.
Fevers associated with many conditions are worse in the evening owing to the circadian rhythm of body temperature; consequently, this pattern need not imply a
true periodic fever. Although fever with acute onset in an otherwise healthy young
adult is usually viral in origin, the presence of shaking chills suggests the possibility of bacteremia; potential sources include pyelonephritis, infection of the biliary
tree, and pneumonia. Other considerations include endocarditis, salmonella infection, typhoid fever, and tularemia. The patient should be asked about any use of
injection drugs and about recent travel, contact with animals, and ingestion of untreated water or unpasteurized milk or milk products. Although the shaking chills
suggest bacteremia, viral illness (e.g., acute infection with the human immunodeficiency virus [HIV]) and noninfectious causes of fever (e.g., cancer or rheumatologic disease) must also be considered. A sexual history should be taken to identify
behaviors associated with an increased risk of HIV infection, with information
obtained regarding the number of sex partners and a history of sex in exchange for
money or illicit drugs or of sex with men.
The patient reported no neck stiffness, arthralgias, morning stiffness, gastrointestinal illness, or weight loss but noted sweats with his fevers. There had been no alterations in mental status. His medical history was notable only for knee arthroscopies;
he had no internal fixation device. The only medication he was taking was acetaminophen. He had not received the influenza vaccine and had a history of rash on treatment with cephalosporins. The patient lived in the Boston area with his wife, who was
his only sexual partner, and there was no history of a sexually transmitted disease. He
did not smoke or use illicit drugs, and he drank one to two alcoholic beverages on
social occasions. He had spent several days in Cape Cod, Massachusetts, 3 weeks
before presentation. His only overseas travel was a trip to Uganda 2 years earlier. He
had received appropriate vaccinations before that trip and reported excellent adherence to malaria prophylaxis.
Given the recent trip to Cape Cod, tickborne illnesses with an incubation period of
1 to 6 weeks must be considered. In the Cape Cod area, the predominant tickborne
n engl j med 371;19nejm.orgnovember 6, 2014
The New England Journal of Medicine
Downloaded from nejm.org on January 20, 2015. For personal use only. No other uses without permission.
Copyright 2014 Massachusetts Medical Society. All rights reserved.
1833
The
n e w e ng l a n d j o u r na l
illnesses are Lyme disease, anaplasmosis, and babesiosis, all of which can cause a nonspecific flulike illness with fever, malaise, myalgias, and
headache. Although the peak incidence typically
occurs from May through August, infections can
occur at any time of year. Lyme disease is characteristically manifested as erythema migrans, a
macular, erythematous rash with central clearing
(known as a bulls-eye rash). In early localized
Lyme disease, the rash develops at the site of the
bite within a few weeks after the bite; multiples
of such lesions, which can be seen in early disseminated Lyme disease, may develop weeks to
months after infection. Although many patients
do not notice the rash, patients for whom there is
a suspicion of Lyme disease should nevertheless
be questioned about any recent rash. Clinicians
should also inquire about activities associated with
tick exposure, such as walking in grassy fields,
wooded areas, or brush.
This patients travel to Uganda merits consideration for infections that can be acquired in East
Africa and are manifested long after acquisition.
These diseases include malaria, tuberculosis,
filariasis (although this infection is unlikely after short-term travel), visceral leishmaniasis, and
Q fever. Malaria relapses are more likely to begin with rigors than are primary infections. The
physical examination should focus on the liver
and spleen (since hepatosplenomegaly can develop in association with malaria, visceral leishmaniasis, or Q fever), the lungs (particularly the
upper lobe, which is often involved in tuberculosis), the heart (since pericardial rubs or murmurs
can be detected in association with Q fever), and
the extremities and the scrotum (since lymphangitis and lymphedema can develop in association
with filarial fevers).
of
m e dic i n e
tender spleen tip was palpable at the left costal border. There was no peripheral edema and no cervical,
axillary, or inguinal lymphadenopathy. The distal
pulses were 2+ bilaterally. Scattered petechiae were
present on the anterior shins and both feet. The
neurologic examination was within normal limits.
The examination is notable for splenomegaly and
petechiae, findings that should be considered in
the context of the patients reported fever. Petechiae may occur with a variety of viral infections
and with sepsis from meningococcal or pneumococcal infections. Noninfectious causes of fever
and petechial rash include thrombotic thrombocytopenic purpura, a condition in which fever and
petechial rash are accompanied by renal failure,
neurologic symptoms, and hemolytic anemia. In
the presence of splenomegaly, however, the petechiae may represent the hemostatic consequences of splenic sequestration of platelets. The differential diagnosis of splenomegaly and fever
includes hematologic cancers, collagen vascular
diseases, and multiple infectious causes, with infectious mononucleosis being the most likely
cause of infection in a young adult. Although cervical lymphadenopathy, which is commonly associated with mononucleosis, is not present,
mononucleosis remains a possibility; a finding
of atypical lymphocytosis on a complete blood
count would support this diagnosis. Other infectious causes of fever and splenomegaly include
viral hepatitis, babesiosis, cytomegalovirus infection, bacterial endocarditis, and malaria.
The basic metabolic panel and liver-function tests
were normal. The white-cell count was 5400 per
cubic millimeter, with 80% neutrophils, 7% lymphocytes, and 5% monocytes. The hemoglobin
level was 11.9 g per deciliter, the hematocrit 35.1%,
and the platelet count 50,000 per cubic millimeter.
Mean corpuscular volume was 84.4 fl. The prothrombin time was 16.3 seconds (normal range,
12.2 to 14.6), partial thromboplastin time 34.7 seconds (normal range, 23.8 to 36.6), and the international normalized ratio 1.3 (normal range, 0.9 to
1.1). The urinalysis was normal. The lactate dehydrogenase level was elevated at 283 U per liter
(normal range, 135 to 225). Rapid screening of a
nasal swab for influenza viruses was negative.
At presentation, the patients temperature was
98.0F (36.7C), his heart rate 90 beats per minute,
blood pressure 121/57 mm Hg, and oxygen saturation 97% while he was breathing ambient air.
He appeared fatigued but was not in acute distress.
There was no scleral icterus or conjunctival pallor.
His oropharynx was clear and without erythema
or exudates. The neck was supple. The jugular venous pressure was 5 cm of water. The heart rate
was regular, with no murmurs, gallops, or rubs,
and the lungs were clear to auscultation. The abdomen was soft and nontender, with normal The normal white-cell count makes severe bactebowel sounds. There was no hepatomegaly. A non- rial infection unlikely. Mild anemia and moderate
1834
n engl j med 371;19nejm.orgnovember 6, 2014
The New England Journal of Medicine
Downloaded from nejm.org on January 20, 2015. For personal use only. No other uses without permission.
Copyright 2014 Massachusetts Medical Society. All rights reserved.
clinical problem-solving
thrombocytopenia may occur with a variety of
viral and parasitic infections. The slight elevation
in the lactate dehydrogenase level is a nonspecific marker that can be seen in a variety of conditions, including hemolytic anemia, cancer, and
infectious mononucleosis. Among the tickborne
illnesses, babesiosis and anaplasmosis remain
considerations. Babesiosis can cause hemolytic
anemia, thrombocytopenia, and splenomegaly,
but it is also often reflected in abnormalities on
liver-function tests. Anaplasmosis causes leukopenia and anemia, thrombocytopenia, and liverfunction abnormalities. Malaria can cause anemia,
thrombocytopenia, and splenomegaly. It would be
helpful to obtain a peripheral-blood smear, which
might reveal intraerythrocytic parasites (such as
those present in babesiosis and malaria), intracytoplasmic inclusions (morulae are seen in 20 to
80% of neutrophils in patients with anaplasmosis), or abnormal myelocyte or lymphocyte lineages
(suggesting hematologic cancer).
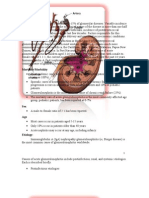
Figure 1. Thin Smear of Peripheral Blood.
The smear shows an enlarged red cell with Schffners
dots (intracytoplasmic granulation) and an intraerythrocytic ring form (short arrow), an ameboid trophozoite in an enlarged red cell (long arrow), and a gametocyte with pigment (arrowhead).
A peripheral-blood smear revealed intraerythro- tation, years after presumed exposure, P. falciparum
cytic parasites.
can be ruled out, since it does not give rise to
relapses. The morphologic features of the parasites
Intraerythrocytic parasites are seen in both ma- and the infected erythrocytes make it possible to
laria and babesiosis. Although Babesia microti is differentiate between two other species, P. vivax
endemic in Cape Cod, infection in late winter or and P. ovale, both of which can cause relapse
early spring is unusual. The patients travel history months or years after initial infection.
places him at risk for exposure to malaria, and
his clinical presentation could be explained by a On further examination of the peripheral-blood
malaria relapse. The peripheral-blood smear smear, Schffners dots (intracytoplasmic granulashould be examined carefully to distinguish be- tion caused by the maturation of P. vivax or P. ovale
tween these infective agents.
within a cell) could be seen, in addition to inPlasmodia metabolize heme to form an intra- traerythrocytic ring forms. More mature ameboid
cellular crystallized pigment, hemozoin. Although trophozoites (Fig. 1) were also detected, as were
hemozoin is not invariably identified in cases of pigment-containing, rounded gametocytes, the
malaria, its presence reliably distinguishes ma- presence of which is consistent with P. vivax. The
laria infection from babesia infection. Malaria parasite burden was low at 0.4%.
parasites can be distinguished from B. microti by
the presence of recognizable gametocytes (char- Parasitemia exceeding 5% is correlated with seacteristically banana-shaped in Plasmodium falci- vere infection. This patients low parasite burden
parum and round, with a granular appearance, in is characteristic of P. vivax. The treatment of manonfalciparum species). The gametocytes are laria is guided by anticipated drug susceptibility.
precursors of the sexual forms of the malaria Whereas P. falciparum from many parts of the
parasite. In addition, intracellular vacuoles and world is chloroquine-resistant, nonfalciparum inextracellular merozoites are unusual in malaria fections from most regions respond well to chlobut common in babesiosis, and the classic Mal- roquine; P. vivax infections acquired in Uganda
tese cross (a tetrad of parasites budding at right would be expected to be chloroquine-sensitive.
angles) is unique to babesia species.
Whereas chloroquine should effectively end this
When malaria is diagnosed, the species should relapse by eliminating parasitemia, it will not
be identified. Given the patients delayed presen- prevent future relapses, since it does not affect
n engl j med 371;19
nejm.org
november 6, 2014
The New England Journal of Medicine
Downloaded from nejm.org on January 20, 2015. For personal use only. No other uses without permission.
Copyright 2014 Massachusetts Medical Society. All rights reserved.
1835
The
n e w e ng l a n d j o u r na l
the latent forms of P. vivax (hypnozoites) in the
liver. A 2-week course of primaquine should also
be prescribed to clear the hepatic reservoir of
malaria, thus preventing future relapses. Given
the risk of hemolysis in patients with glucose6-phosphate dehydrogenase (G6PD) deficiency who
receive treatment with primaquine, the patient
should be tested for G6PD deficiency.
The patient was given 1 g of chloroquine phosphate, followed by 500 mg at 6, 24, and 48 hours.
The fevers and headaches resolved within 12 hours
after initiation of treatment. A repeat blood smear
at 24 hours showed only two gametocytes per
smear. A test for G6PD deficiency was negative.
After receiving the four doses of chloroquine, the
patient was directed to take 30 mg of primaquine
daily for 14 days. At a 2-week follow-up visit, he
remained afebrile and had no residual symptoms
except for some mild fatigue. A repeat peripheralblood smear obtained at that time showed no indication of parasitemia.
C om men ta r y
In hindsight, it is tempting to refer to the patients
fever as cyclic, given the classic association of
cyclic (periodic, or relapsing) fever with malaria.
Although the differential diagnosis for periodic
fever is different from that for randomly recurring fever,1 many febrile illnesses follow a pattern
of nightly fevers with morning defervescence. In
malaria, the febrile pattern is initially irregular
but subsequently regularizes if a dominant brood
of synchronously replicating parasites develops.
Typically, the periodicity of fever is 72 hours with
P. malariae and 48 hours with other species. However, any fever in a patient who has had possible
exposure to malaria should prompt consideration of this diagnosis.
In clinical practice, malaria is often categorized as falciparum versus nonfalciparum (which
includes P. vivax, P. ovale, and P. malariae). P. falciparum carries the highest risk of death. Among
the nonfalciparum species, P. vivax accounts for
the greatest number of cases, and among all species it has the widest geographic range. P. vivax
occurs widely in the tropics, is present in some
temperate areas, and occurs only rarely in subSaharan Africa.2,3 In the United States, the annual
incidence of malaria is approximately 1500 cases.4 In 2010, a total of 1691 cases were reported
to the Centers for Disease Control and Preven1836
of
m e dic i n e
tion (CDC), the largest number reported since
1980; P. falciparum, P. vivax, P. malariae, and P. ovale
were identified in 58%, 19%, 2%, and 2% of
cases, respectively.5
The malaria life cycle at initial infection is
identical across species, beginning with inoculation by a feeding female anopheles mosquito of
immature malaria parasites (sporozoites) that
migrate through the hosts bloodstream to the
liver, where they invade hepatocytes and replicate
by means of schizogony (asexual division into
multinucleated cells called schizonts that contain many merozoites). Mature schizonts rupture
the hepatocyte, releasing tens of thousands of
merozoites into the bloodstream, where they invade erythrocytes and undergo another round of
schizogony, forming trophozoites (the form taken during the feeding stage of the parasite).3
Trophozoites develop into blood-stage schizonts
that usually contain up to 24 merozoites, which,
upon rupture of the erythrocyte, enter the bloodstream and infect other erythrocytes. Fever, rigors,
and other symptoms of the malaria paroxysm are
the consequence of red-cell rupture and release of
merozoites.3
In the case of P. vivax and P. ovale, some sporozoites do not replicate immediately when they
invade hepatocytes but remain dormant (as hypnozoites) for prolonged periods before undergoing schizogony and giving rise to a clinical relapse,6 as occurred in this case. The average time
to relapse is approximately 9 months, but it can
range from weeks to years. The interval to relapse depends on the strain (earlier with tropical
strains and later with temperate strains), the initial inoculum, and host factors (e.g., febrile illnesses can trigger relapse associated with P. vivax).7
None of the commonly used prophylactic agents
(chloroquine, mefloquine, doxycycline, or atovaquoneproguanil) eliminate hypnozoites. Primaquine, the only effective drug against dormant
hypnozoites, has not been approved by the Food
and Drug Administration for primary prophylaxis,8 but the CDC endorses its use for prophylaxis in Latin American countries where P. vivax
predominates, because the drug can prevent both
primary attacks and relapses caused by all species that are a source of malarial infection.9 It is
unlikely that this patient had received primaquine for malaria prophylaxis. Instead, his prophylactic therapy protected him from a clinically
apparent initial infection but did not prevent
dormant infection in his liver.
n engl j med 371;19nejm.orgnovember 6, 2014
The New England Journal of Medicine
Downloaded from nejm.org on January 20, 2015. For personal use only. No other uses without permission.
Copyright 2014 Massachusetts Medical Society. All rights reserved.
clinical problem-solving
In regions where malaria is endemic, the risk
of disease can be reduced by limiting mosquito
bites; it is helpful to wear protective clothing,
apply repellents such as N,N-diethyl-3-methylbenzamide (DEET) or picaridin to skin and
permethrin to clothing, sleep under insecticidetreated bed nets, and avoid outdoor activities during peak biting times (in most areas, between
dusk and dawn).10 Even with these measures,
chemoprophylaxis is essential for travelers to
areas in which the disease is endemic. Owing to
the widespread resistance of P. falciparum to chloroquine, the main agents for chemoprophylaxis
are mefloquine and doxycycline, which are active
against bloodstream parasites and prevent clinical disease, and atovaquoneproguanil, which is
active against parasites in the liver and the bloodstream but not against hypnozoites. The choice
of drug must take into account the travel destination and duration of stay, concurrent medical
conditions, adverse-event profile, and cost.
In patients with acute or recurrent malaria
infection, treatment depends on the species and
the resistance status in the area where the infection was acquired. P. falciparum is resistant to
chloroquine in most regions in which it is endemic and resistant to mefloquine in parts of
Southeast Asia. In contrast, nonfalciparum malaria parasites do not have substantial resistance
to mefloquine, and the distribution of chloroquine-resistant P. vivax malaria is limited, occurReferences
1. Long SS. Distinguishing among prolonged, recurrent, and periodic fever syndromes: approach of a pediatric infectious diseases subspecialist. Pediatr Clin
North Am 2005;52:811-35.
2. Maguire JD, Baird JK. The non-falciparum malarias: the roles of epidemiology, parasite biology, clinical syndromes,
complications and diagnostic rigour in
guiding therapeutic strategies. Ann Trop
Med Parasitol 2010;104:283-301.
3. Dhorda M, Nyehangane D, Rnia L,
Piola P, Guerin PJ, Snounou G. Transmission of Plasmodium vivax in south-western Uganda: report of three cases in pregnant women. PLoS One 2011;6(5):e19801.
4. Malaria facts. Atlanta: Centers for
ring primarily in Indonesia and Papua New Guinea.11 After treatment is initiated, peripheral-blood
smears should be obtained daily for 4 days (parasitemia is typically eliminated by day 4), on days
7 and 28 to confirm eradication, and at any time
symptoms recur, suggesting treatment failure.10
In areas other than those with known chloroquine resistance, chloroquine, followed by a 14-day
course of primaquine to prevent subsequent relapses, remains the standard treatment for P. vivax
parasitemia.2 Among patients with a contraindication to primaquine therapy (e.g., pregnant women or persons with G6PD-deficiency), treatment
with chloroquine alone carries a 20% risk of relapse12; extended chloroquine prophylaxis can be
offered to patients who have frequent relapses.
This case highlights the importance of travel
history (recent and remote) in constructing the
differential diagnosis of a febrile illness. Although babesiosis was a possibility in this patient, since he had traveled only 3 weeks before
presentation to an area in which the disease is
endemic, consideration of the possibility of relapse of P. vivax malaria years after initial exposure prompted careful review of the blood smear
and led quickly to the correct diagnosis and appropriate therapy.
No potential conflict of interest relevant to this article was
reported.
Disclosure forms provided by the authors are available with
the full text of this article at NEJM.org.
We thank Dr. James Maguire for his clinical insights.
Disease Control and Prevention (http://
www.cdc.gov/malaria/about/facts.html).
5. Malaria surveillance United States,
2010. MMWR Surveill Summ 2012;61(2):
1-17.
6. Mueller I, Galinski MR, Baird JK, et
al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria
parasite. Lancet Infect Dis 2009;9:55566.
7. White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria.
Malar J 2011;10:297.
8. Chen LH, Wilson ME, Schlagenhauf P.
Controversies and misconceptions in malaria chemoprophylaxis for travelers.
JAMA 2007;297:2251-63.
9. Steinhardt LC, Magill AJ, Arguin PM.
Review: malaria chemoprophylaxis for
travelers to Latin America. Am J Trop Med
Hyg 2011;85:1015-24.
10. Freedman DO. Malaria prevention in
short-term travelers. N Engl J Med 2008;
359:603-12.
11. Baird JK. Chloroquine resistance in
Plasmodium vivax. Antimicrob Agents
Chemother 2004;48:4075-83.
12. Griffith KS, Lewis LS, Mali S, Parise
ME. Treatment of malaria in the United
States: a systematic review. JAMA 2007;
297:2264-77.
Copyright 2014 Massachusetts Medical Society.
clinical problem-solving series
The Journal welcomes submissions of manuscripts for the Clinical Problem-Solving
series. This regular feature considers the step-by-step process of clinical decision
making. For more information, please see authors.NEJM.org.
n engl j med 371;19nejm.orgnovember 6, 2014
The New England Journal of Medicine
Downloaded from nejm.org on January 20, 2015. For personal use only. No other uses without permission.
Copyright 2014 Massachusetts Medical Society. All rights reserved.
1837
You might also like
- A Chilly FeverDocument5 pagesA Chilly FeverChangNo ratings yet
- Home Sweet HomeDocument6 pagesHome Sweet HomeMr. LNo ratings yet
- Child With Aseptic Meningitis: Diagnosis and TreatmentDocument4 pagesChild With Aseptic Meningitis: Diagnosis and TreatmentCheng XinvennNo ratings yet
- Dengue CurrentDocument6 pagesDengue CurrentDani BurgosNo ratings yet
- A Pain in The NeckDocument6 pagesA Pain in The NeckBudi IstriawanNo ratings yet
- LepsDocument4 pagesLepslynharee100% (1)
- Stemming The Outbreak: SyphilisDocument7 pagesStemming The Outbreak: SyphilisamimusriNo ratings yet
- West Nile virus causes fever, encephalitis, rarely fatalDocument7 pagesWest Nile virus causes fever, encephalitis, rarely fatalkojerzooNo ratings yet
- Clinical Presentation: by Dr. Raffiq AbbasDocument36 pagesClinical Presentation: by Dr. Raffiq AbbasKarthick UnleashNo ratings yet
- Spiraling Out of Control: Clinical Problem-SolvingDocument6 pagesSpiraling Out of Control: Clinical Problem-SolvingjjjkkNo ratings yet
- Anak 1Document4 pagesAnak 1Vega HapsariNo ratings yet
- Art2 Eq6 PDFDocument6 pagesArt2 Eq6 PDFmaky yrigoyenNo ratings yet
- Ni Hms 26626Document4 pagesNi Hms 26626qammarNo ratings yet
- Emerging Infectious Diseases and EpidemiologyDocument25 pagesEmerging Infectious Diseases and EpidemiologyAnika Yasmin 2012965048No ratings yet
- Case 06Document5 pagesCase 06Chefera AgaNo ratings yet
- Etiologies of Fever of Unknown Origin in Adults - UpToDate (2019) PDFDocument17 pagesEtiologies of Fever of Unknown Origin in Adults - UpToDate (2019) PDFMoisés León RuizNo ratings yet
- Fever of Unknown OriginDocument8 pagesFever of Unknown OriginReyhan HadimanNo ratings yet
- Prolonged Fever, Hepatosplenomegaly, and Pancytopenia in A 46-Year-Old WomanDocument6 pagesProlonged Fever, Hepatosplenomegaly, and Pancytopenia in A 46-Year-Old WomanFerdy AlviandoNo ratings yet
- 6a. Diarrea Por SalmonellaDocument7 pages6a. Diarrea Por SalmonellaSantiago MiñanoNo ratings yet
- HSV2Document5 pagesHSV2daniela_chrc9100No ratings yet
- Henoch Schoenlein PurpuraDocument10 pagesHenoch Schoenlein PurpuraAlberto Kenyo Riofrio PalaciosNo ratings yet
- Crux 05Document8 pagesCrux 05Rajkishor YadavNo ratings yet
- A Crazy Cause of DyspneaDocument6 pagesA Crazy Cause of DyspneaAbdur Rachman Ba'abdullahNo ratings yet
- Jurnal MalariaDocument9 pagesJurnal MalarianabilaNo ratings yet
- Meningitis (Physical Exam)Document6 pagesMeningitis (Physical Exam)MohammadAwitNo ratings yet
- DengueDocument30 pagesDengueSaurav AroraNo ratings yet
- Nejmcps 1808494Document6 pagesNejmcps 1808494dias100% (1)
- 8.3 Medicine - Tropical Infectious Diseases Leptospirosis 2014ADocument7 pages8.3 Medicine - Tropical Infectious Diseases Leptospirosis 2014ABhi-An BatobalonosNo ratings yet
- Typhoid FeverDocument47 pagesTyphoid FeverSujatha J Jayabal100% (2)
- Case Report: Fatal Staphylococcal Infection Following Classic Dengue FeverDocument4 pagesCase Report: Fatal Staphylococcal Infection Following Classic Dengue FeverRia Septi HarmiaNo ratings yet
- Yellow Fever Literature ReviewDocument7 pagesYellow Fever Literature Revieweowcnerke100% (1)
- Anatomy & Medicine MCQs April 14Document21 pagesAnatomy & Medicine MCQs April 14sb medexNo ratings yet
- Acute Fulminant Leptospirosis with Multi-Organ FailureDocument4 pagesAcute Fulminant Leptospirosis with Multi-Organ FailureMuhFarizaAudiNo ratings yet
- Thyfoid Fever: A Diagnostic ChallengeDocument36 pagesThyfoid Fever: A Diagnostic ChallengeYogie DjNo ratings yet
- Review Article: Etiology and Risk Factors of Febrile Seizure - An UpdateDocument10 pagesReview Article: Etiology and Risk Factors of Febrile Seizure - An UpdateResty Rahmiliah RahimNo ratings yet
- Typhoid Fever: David T Wheeler, MDDocument14 pagesTyphoid Fever: David T Wheeler, MDChandra KushartantoNo ratings yet
- Human Brucellosis: John M. Sauret, MD, and Natalia Vilissova, MDDocument6 pagesHuman Brucellosis: John M. Sauret, MD, and Natalia Vilissova, MDAndika Budi KurniantoNo ratings yet
- Arthropod-Borne Encephalitides - UpToDateDocument21 pagesArthropod-Borne Encephalitides - UpToDateBhargav YagnikNo ratings yet
- Lupus Primary CareDocument22 pagesLupus Primary Carewoopdeedoo903No ratings yet
- Noro VirusDocument32 pagesNoro VirusSCIH HFCPNo ratings yet
- Case Study #1 Rough NotesDocument6 pagesCase Study #1 Rough NotesChantal CarnesNo ratings yet
- Viral MyocarditisDocument42 pagesViral MyocarditisAlishba AtifNo ratings yet
- Practice Essentials: 11 Travel Diseases To Consider Before and After The TripDocument12 pagesPractice Essentials: 11 Travel Diseases To Consider Before and After The TripGio Vano NaihonamNo ratings yet
- Final Paper Rheumatic Heart DiseaseDocument16 pagesFinal Paper Rheumatic Heart DiseasePrincess TumambingNo ratings yet
- Lapsus Tifoid + DengueDocument10 pagesLapsus Tifoid + DengueM.ThaufiqurrakhmanNo ratings yet
- Dengue Hemorrhagic Fever and Acute Hepatitis: A Case ReportDocument4 pagesDengue Hemorrhagic Fever and Acute Hepatitis: A Case ReportKamilah NasarNo ratings yet
- Clin Infect Dis. 2003 Stauffer 1340 8Document9 pagesClin Infect Dis. 2003 Stauffer 1340 8MD Luthfy LubisNo ratings yet
- Sickle Cell DiseaseDocument10 pagesSickle Cell Diseasekarthikavvari2002No ratings yet
- What Is LeptospirosisDocument18 pagesWhat Is LeptospirosisLuphly TaluvtaNo ratings yet
- Henoch Schonlein Purpura (IgA Vasculitis)Document15 pagesHenoch Schonlein Purpura (IgA Vasculitis)Emily Eresuma100% (1)
- RigorsDocument3 pagesRigorsLina HasbiNo ratings yet
- Obeagu Emmanuel IfeanyiDocument5 pagesObeagu Emmanuel Ifeanyishoukat aliNo ratings yet
- Human Immunodeficiency Virus (Hiv) & Sexually Transmitted Infections (Sti)Document37 pagesHuman Immunodeficiency Virus (Hiv) & Sexually Transmitted Infections (Sti)Nicola4No ratings yet
- DiagnosisDocument6 pagesDiagnosisZackNo ratings yet
- Partial of PathophysiologyDocument56 pagesPartial of Pathophysiologytoolsdesk1No ratings yet
- Istory: Pneumoniae MeningitisDocument2 pagesIstory: Pneumoniae MeningitisMohammadAwitNo ratings yet
- Epidemiology: Glomerulonephritis Represents 10-15% of Glomerular Diseases.Document16 pagesEpidemiology: Glomerulonephritis Represents 10-15% of Glomerular Diseases.kc_lhiraNo ratings yet
- Fever and PetechiaeDocument9 pagesFever and PetechiaeJohn Carlo PizarraNo ratings yet
- Fever of Unknown OriginDocument6 pagesFever of Unknown OriginJyothsna S MandarapuNo ratings yet
- Cognitiveimpairmentand Dementiainparkinson Disease: Jennifer G. Goldman,, Erica SiegDocument13 pagesCognitiveimpairmentand Dementiainparkinson Disease: Jennifer G. Goldman,, Erica SiegJuan Carlos Carbajal SilvaNo ratings yet
- Session 539000 4 PDFDocument16 pagesSession 539000 4 PDFJuan Carlos Carbajal SilvaNo ratings yet
- PTSD in the Elderly: Understanding Risk Factors and TreatmentDocument11 pagesPTSD in the Elderly: Understanding Risk Factors and TreatmentJuan Carlos Carbajal SilvaNo ratings yet
- Transcription 539000 3 PDFDocument8 pagesTranscription 539000 3 PDFJuan Carlos Carbajal SilvaNo ratings yet
- Session 539000 4 PDFDocument16 pagesSession 539000 4 PDFJuan Carlos Carbajal SilvaNo ratings yet
- Cancer Cachexia: Subtitles and TranscriptionsDocument9 pagesCancer Cachexia: Subtitles and TranscriptionsJuan Carlos Carbajal SilvaNo ratings yet
- Transcription 539000 3 PDFDocument8 pagesTranscription 539000 3 PDFJuan Carlos Carbajal SilvaNo ratings yet
- Session 539000 4 PDFDocument16 pagesSession 539000 4 PDFJuan Carlos Carbajal SilvaNo ratings yet
- Session 539000 4 PDFDocument16 pagesSession 539000 4 PDFJuan Carlos Carbajal SilvaNo ratings yet
- ¿Existe Correlación Entre El Nivel de Vitamina D y El Desarrollo de Lupus PDFDocument11 pages¿Existe Correlación Entre El Nivel de Vitamina D y El Desarrollo de Lupus PDFJuan Carlos Carbajal SilvaNo ratings yet
- Transcription 539000 3 PDFDocument8 pagesTranscription 539000 3 PDFJuan Carlos Carbajal SilvaNo ratings yet
- Chan 2019Document10 pagesChan 2019Juan Carlos Carbajal SilvaNo ratings yet
- Guia Holandesa Crisis HtaDocument8 pagesGuia Holandesa Crisis HtaJuancamilo Ocampo MartinezNo ratings yet
- Vasopresores en Emergencia PDFDocument28 pagesVasopresores en Emergencia PDFJuan Carlos Carbajal SilvaNo ratings yet
- Embolismo Pulmonar Manejo 2016Document15 pagesEmbolismo Pulmonar Manejo 2016Karen Grissell Serrano RamosNo ratings yet
- Nicol Le 201699Document16 pagesNicol Le 201699Juan Carlos Carbajal SilvaNo ratings yet
- ¿Existe Correlación Entre El Nivel de Vitamina D y El Desarrollo de Lupus PDFDocument11 pages¿Existe Correlación Entre El Nivel de Vitamina D y El Desarrollo de Lupus PDFJuan Carlos Carbajal SilvaNo ratings yet
- 10 1097@CCM 0000000000000625Document8 pages10 1097@CCM 0000000000000625Juan Carlos Carbajal SilvaNo ratings yet
- Bartonelosis Carrions Disease in The Pediatric Population of Peru An Overview and UpdaDocument9 pagesBartonelosis Carrions Disease in The Pediatric Population of Peru An Overview and UpdaJuan Carlos Carbajal SilvaNo ratings yet
- Monocular Diplopia Binocular DiplopiaDocument11 pagesMonocular Diplopia Binocular DiplopiaPomtungNo ratings yet
- Masonry TutorialDocument45 pagesMasonry TutorialGajendra Joshi100% (2)
- MULTIPLE CHOICE-ComputationalDocument5 pagesMULTIPLE CHOICE-Computationaljie calderonNo ratings yet
- Pathway Microtomy and Paraffin Section PreparationDocument35 pagesPathway Microtomy and Paraffin Section PreparationRia CanteroNo ratings yet
- Sepam 100 LD PresentationDocument11 pagesSepam 100 LD Presentationalisann87No ratings yet
- Chemistry Higher Level Paper 3: Instructions To CandidatesDocument40 pagesChemistry Higher Level Paper 3: Instructions To CandidatesAleksander Ziolkowski100% (1)
- Stages of Psychosocial DevelopmentDocument5 pagesStages of Psychosocial DevelopmentKafitaNo ratings yet
- WP104 PDFDocument33 pagesWP104 PDFDiah wuri andaniNo ratings yet
- Unsteady State Heat and Mass TransferDocument14 pagesUnsteady State Heat and Mass Transfernhalieza1067No ratings yet
- Control Panels CP405 Control Panels CP408: Operating InstructionsDocument23 pagesControl Panels CP405 Control Panels CP408: Operating InstructionsHitesh PanigrahiNo ratings yet
- Biological Molecules Notes o LevelDocument10 pagesBiological Molecules Notes o LevelFangsNo ratings yet
- MST125-BbCc ASSIGNMENTDocument13 pagesMST125-BbCc ASSIGNMENTFaye ManaulNo ratings yet
- uPVC Windows Indian StandardDocument61 pagesuPVC Windows Indian StandardRamachandra Budihal71% (7)
- Safety Data Sheet: 1. Chemical Product and Company IdentificationDocument6 pagesSafety Data Sheet: 1. Chemical Product and Company IdentificationNguyễn Thị ThúyNo ratings yet
- Final Study SS of 2nd PB 2023Document65 pagesFinal Study SS of 2nd PB 2023rahul SNo ratings yet
- Cell Counting Neubauer ChamberDocument6 pagesCell Counting Neubauer ChamberHerma ZulaikhaNo ratings yet
- User Manual of YKD-9122-2022.6Document18 pagesUser Manual of YKD-9122-2022.6Dr Ibrahima BaldeNo ratings yet
- Project Guide: C7.1 Marine Propulsion EngineDocument62 pagesProject Guide: C7.1 Marine Propulsion EngineAhmad Arrisal AttaufiqulNo ratings yet
- Sous Vide Cooking ChartDocument3 pagesSous Vide Cooking ChartChefCristian100% (1)
- Bexley Selection Tests Specimen Questions - Verbal ReasoningDocument8 pagesBexley Selection Tests Specimen Questions - Verbal Reasoningpflora41No ratings yet
- (Ebook) - Piers Anthony - GhostDocument116 pages(Ebook) - Piers Anthony - GhostChandresh KothariNo ratings yet
- Sample Waste Management Plan: Appendix 3.1Document9 pagesSample Waste Management Plan: Appendix 3.1Tendou ZulNo ratings yet
- Entrepreneurship Chapter 9 MCQsDocument4 pagesEntrepreneurship Chapter 9 MCQsTooba0% (1)
- OSHA AssignmentDocument3 pagesOSHA AssignmentJoashYenNo ratings yet
- Dulangan National High School: Office of The Guidance CenterDocument3 pagesDulangan National High School: Office of The Guidance CenteraneworNo ratings yet
- Promotion of Tax Culture in Pakistan: Perspective, Prospects and ChallengesDocument5 pagesPromotion of Tax Culture in Pakistan: Perspective, Prospects and ChallengesRaheel JoyiaNo ratings yet
- Chapter 2 LaboratoryDocument6 pagesChapter 2 LaboratoryVea Roanne GammadNo ratings yet
- BSNL telephone bill detailsDocument2 pagesBSNL telephone bill detailsNitikaNo ratings yet
- G8D Process: A Methodical 8-Step Problem Solving ToolDocument34 pagesG8D Process: A Methodical 8-Step Problem Solving Toolpkrganesh100% (1)
- Guidelines For The Design and Construction of Health Care FacilitiesDocument370 pagesGuidelines For The Design and Construction of Health Care Facilitiesmasoodae93% (15)