Professional Documents
Culture Documents
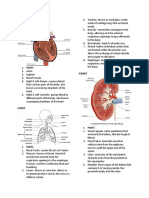
Anti D
Uploaded by
qweeyip10210 ratings0% found this document useful (0 votes)
39 views1 pageANTI-D IgG / IgM BLENDED MONOCLONAL RHESUS TYPING REAGENT Principle: The test is based on the principle of agglutination. The IgG anti-D directly agglutinates D positive red cells, including the majority of D variants, with the exception of DVI and low grade weak D (Du) phenotypes.
Original Description:
Original Title
Anti D
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentANTI-D IgG / IgM BLENDED MONOCLONAL RHESUS TYPING REAGENT Principle: The test is based on the principle of agglutination. The IgG anti-D directly agglutinates D positive red cells, including the majority of D variants, with the exception of DVI and low grade weak D (Du) phenotypes.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
39 views1 pageAnti D
Uploaded by
qweeyip1021ANTI-D IgG / IgM BLENDED MONOCLONAL RHESUS TYPING REAGENT Principle: The test is based on the principle of agglutination. The IgG anti-D directly agglutinates D positive red cells, including the majority of D variants, with the exception of DVI and low grade weak D (Du) phenotypes.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
TUBE, SLIDE AND MICROPLATE TESTS
STORE AT 2 – 8°C
ANTI-D IgG / IgM
BLENDED MONOCLONAL RHESUS TYPING REAGENT FOR IN-VITRO DIAGNOSTIC USE ONLY
Principle: stream. Positive reactions remain as distinct buttons either on
The test is based on the principle of agglutination. Red cells which the bottom of the well or occasionally sliding down the side.
the antigen will agglutinate when tested against the corresponding 7. For apparently negative results which are to be tested for DVI and
antibody. other weak D phenotypes, retest using the indirect antiglobulin
test procedure.
Presentation: Note: If performing indirect antiglobulin tests by validated microplate
Reagent Code Size techniques, tests should be washed a minimum of 3 to 4 times due to
the small volume of saline which can be added to each microwell.
Anti-D Blend BGD00010 10 ml
Slide Technique:
1. Prepare a 35 – 45% red cell suspension using either their own
Composition: The Anti-D IgG / IgM Blend has been prepared from
group or group compatible plasma or serum.
carefully blended monoclonal IgM and IgG anti-D’s. The IgG anti-D
2. Onto a slide which is at room temperature (18 – 25°C) place 1
directly agglutinates D positive red cells, including the majority of D
volume of Anti-D Blend and 1 volume of the 35 – 45% Red Cell
variants, with the exception of DVI , and a high proportion of weak D
Suspension. Note that stronger reactions will be observed if
(Du) phenotypes. The IgG anti-D agglutinates DVI and low grade weak
testing is done on a slide at between 40°C and 50°C.
D (Du) phenotypes by the indirect antiglobulin test method. The
3. Using a clean applicator stick, mix the reagent and cell
antibodies are diluted in a phosphate buffer which contains sodium
suspension over an area of approximately 20 x 40 mm.
chloride, bovine albumin and macromolecular potentiators to give a
4. Slowly tilt the slide back and forth for no longer than two minutes
reagent which is optimised for use in tube, slide or microplate tests.
and observe for signs of agglutination. Record the results.
Contains 0.1% sodium azide.
5. For apparently negative results which are to be tested for DVI and
other weak D phenotypes, retest using the indirect antiglobulin
Although all our components which have been derived from human
test procedure.
origin have been tested and found to be negative for the presence of
anti-HIV, anti-HCV as well as HbsAg, it is recommended that they be Indirect Antiglobulin Test:
handled cautiously and treated potentially infectious. 1. Prepare a 2-3% red cell suspension using isotonic buffered
saline with a pH of 6.8 – 7.2.
Storage:
2. Place in a glass test tube 1 volume of Anti-D Blend and 1 volume
• Store components at 2-8°C.
of the Red Cell Suspension.
• Do not freeze or expose to elevated temperatures. 3. Mix well and incubate at 37°C for 15 – 30 minutes.
• Do not use beyond the expiry date. 4. After incubation wash the cells once in isotonic buffered saline,
• Marked turbidity may indicate reagent contamination or decanting the saline completely.
deterioration. 5. Add two volumes of polyspecific anti-human globulin or anti-IgG
to the dry cell button and mix gently to resuspend the cells.
Samples:
6. Centrifuge at 900 – 1000 rcf for 15 seconds.
• Blood samples which have been drawn with or without anti-
7. Gently resuspend the cells and examine macroscopically for
coagulant may be used.
signs of agglutination. Record the results.
• Testing should be performed as soon as possible to avoid false 8. Confirm validity of negative tests with IgG sensitised cells.
reactions occurring due to contamination or incorrect storage.
• Samples may be stored at between 2 and 8°C and tested within Reaction Stability: Following centrifugation all tube and microplate
two days provided there is no evidence of haemolysis. tests should be read immediately and results interpreted without
• Samples collected into EDTA or Heparin should be tested within delay. Slide tests should be interpreted at the end of two minutes.
48 hours, clotted samples within 14 days and those drawn into
ACD, CPD or CPDA-1 up to their expiry dates or within 3 days of Quality Control:
withdrawal. • It is recommended that appropriate antigen-positive and antigen-
• Prolonged storage of red cells may result in weaker reactions. negative cells be tested with the reagents on each day of use in
• Bacterial contamination may cause false test results. order to confirm the reactivity and specificity of the blood
grouping reagents.
Test Procedures:
Tube Technique: Limitations of the Procedure:
1. Prepare a 2-3% red cell suspension using isotonic buffered • False positive and negative results may occur due to
saline with a pH of 6.8 – 7.2. contamination of test materials, improper cell concentrations,
2. Place in a glass test tube 1 volume of Anti-D Blend and 1 volume incorrect centrifugation, incubation and temperature times.
of the Red Cell Suspension. • Any deviation from the test procedure could result in inaccurate
3. Mix well and incubate at room temperature (18 – 25°C) for 1 results.
minute. Incubation may be extended to 15 minutes to improve • Stronger direct reactions with weak D phenotypes will be
the detection rate of weak D (Du) phenotypes. observed in tube tests than in slide and microplate tests.
4. Centrifuge at 900 to 1000 rcf for 15 seconds. • Red cells showing a positive direct antiglobulin test cannot be
5. Gently resuspend the cell button and examine macroscopically typed by the indirect antiglobulin test.
for signs of agglutination and record the results. • Weaker reactions may be observed with stored blood.
6. For apparently negative results which are to be tested for DVI and
other weak D phenotypes, proceed to step 3 of the indirect Bibliography:
antiglobulin test. Kohler G., Milstein C. (1975) Continuous culture of fused cells
secreting ab of predefined specificity. Nature 256, 49497
Microplate Technique: Walker RH., ed. Technical Manual, 11th Ed. Bethesda, MD: American
1. Prepare a 2 – 3 % Red Cell Suspension using isotonic buffered Association of Blood Banks, 1993: Ch11
saline with a pH of 6.8 – 7.2. Issitt PD., Applied Blood Group Serology, 3rd Ed. Miami: Montgomery
2. Place in the appropriate well of a U-bottom microplate 1 volume Scientific, 1985. Ch. 10
(30 - 50µl) of ABO Grouping Reagent and 1 volume of the 2 – 3 Jones j., Scott ML., Voak D. Monoclonal anti-D specificity and Rh D
% (30 - 50µl) Red Cell Suspension. structure: criteria for selection of monoclonal anti=D reagents for
3. Mix well, preferably using a microplate shaker, taking care to routine typing of patients and donors. Transfusion Medicine 1995: 5,
avoid cross-well contamination. 171 – 184.
4. Incubate at room temperature (18 – 25°C) for 1 – 15 minutes. Tippett P. Sub-Divisions of the Rh (D) antigen. Med. Lab. Sci. 1988.
5. Centrifuge the microplate at 140rcf for 1 minute. 45. 88-93.
6. Tilt the plate at an angle of 60 – 90° to the bench top and Guidelines for compatibility testing in hospital blood banks. Cli. Lab.
observe over the next three minutes for signs of streaming. Haem., 1987: 9:333-341
Negative reactions allow the cells to flow downwards in a uniform
Reviewed: April 2000
You might also like
- Direct Oral Anticoagulants From Pharmacology To Clinical PracticeDocument283 pagesDirect Oral Anticoagulants From Pharmacology To Clinical PracticesunhaolanNo ratings yet
- The Chemistry of The BloodDocument29 pagesThe Chemistry of The Bloodenemb0% (1)
- Antihuman Globulin (Ahg) TestDocument38 pagesAntihuman Globulin (Ahg) TestJerome ValerianoNo ratings yet
- Essentials of ABO -Rh Grouping and Compatibility Testing: Theoretical Aspects and Practical ApplicationFrom EverandEssentials of ABO -Rh Grouping and Compatibility Testing: Theoretical Aspects and Practical ApplicationRating: 5 out of 5 stars5/5 (1)
- Antiglobulin Test TechniquesDocument26 pagesAntiglobulin Test TechniquesJennifer DixonNo ratings yet
- Scalar Energy PendantDocument12 pagesScalar Energy PendantNicole WeatherleyNo ratings yet
- Blood Banking Tests and ProceduresDocument174 pagesBlood Banking Tests and ProceduresMarydith Ortillo100% (1)
- Reactions of Antibodies with Soluble Antigens: Methods in Immunology and Immunochemistry, Vol. 3From EverandReactions of Antibodies with Soluble Antigens: Methods in Immunology and Immunochemistry, Vol. 3No ratings yet
- Mindray BC 5130 PDFDocument2 pagesMindray BC 5130 PDFAniket Dubey50% (2)
- Capillary Puncture Equipment and Procedures: Topic 7Document39 pagesCapillary Puncture Equipment and Procedures: Topic 7Angelica Camille B. AbaoNo ratings yet
- Health Harmony eDocument181 pagesHealth Harmony eVenkateswara Rao Boda100% (1)
- Anti-HG Eng Rev04Document2 pagesAnti-HG Eng Rev04Ventas2lp IcerlabNo ratings yet
- Direct Antiglobulin TestDocument4 pagesDirect Antiglobulin TestCattrainuhNo ratings yet
- Rh Grouping Techniques and Common ErrorsDocument7 pagesRh Grouping Techniques and Common Errorsimran243No ratings yet
- Anti-D TUEV-CE Rev02Document2 pagesAnti-D TUEV-CE Rev02Ventas2lp IcerlabNo ratings yet
- ANTIBODY SCREENING - FinalDocument4 pagesANTIBODY SCREENING - FinalHaniya KhanNo ratings yet
- Coombs TestDocument17 pagesCoombs Testnativeman2221No ratings yet
- Coomb's Test GuideDocument26 pagesCoomb's Test Guidelubna aloshibiNo ratings yet
- BSA Eng Rev021Document2 pagesBSA Eng Rev021Ventas2lp IcerlabNo ratings yet
- Dialab Monoclonal Blood Grouping ReagentsDocument2 pagesDialab Monoclonal Blood Grouping ReagentsVentas2lp IcerlabNo ratings yet
- RH Blood GroupDocument18 pagesRH Blood GroupKhalid AbdullahNo ratings yet
- COVID-19: Instructions For UseDocument2 pagesCOVID-19: Instructions For UseTheresia IlyanNo ratings yet
- Methods for Detecting Weak Blood Group Antigens and AntibodiesDocument59 pagesMethods for Detecting Weak Blood Group Antigens and AntibodiesYuliyanti YasinNo ratings yet
- Coombs FinalDocument32 pagesCoombs FinalMedicine 0786No ratings yet
- Aso Slide TestDocument16 pagesAso Slide TestMegasonNo ratings yet
- Rubella IgG 1301Z-WebDocument6 pagesRubella IgG 1301Z-WebvolvoproNo ratings yet
- The Antiglobulin Test (Direct & Indirect) : CompanyDocument16 pagesThe Antiglobulin Test (Direct & Indirect) : CompanyAirline Tourism and Hospitality Management KMGGPGCNo ratings yet
- The Antiglobulin Test (Coombs TestDocument23 pagesThe Antiglobulin Test (Coombs TestMarj Mendez100% (1)
- COVID-19: Instructions For UseDocument2 pagesCOVID-19: Instructions For UseAKBAR SAPUTRANo ratings yet
- CEPI044A RPR Carbon Kit Issue 06 IFUDocument2 pagesCEPI044A RPR Carbon Kit Issue 06 IFUslh labNo ratings yet
- Coombs Anti-Igg: Id-Card Direct and Indirect Antiglobulin TestingDocument2 pagesCoombs Anti-Igg: Id-Card Direct and Indirect Antiglobulin TestingVivek PatelNo ratings yet
- Total Ige Elisa KitDocument2 pagesTotal Ige Elisa KitvaniaNo ratings yet
- Dengue IgMDocument2 pagesDengue IgMMaherNo ratings yet
- Interpretation of ResultDocument2 pagesInterpretation of ResultNatural Science BiologyNo ratings yet
- Total IgEDocument2 pagesTotal IgEMaherNo ratings yet
- RPR Test for Syphilis DetectionDocument6 pagesRPR Test for Syphilis DetectionREMAN ALINGASANo ratings yet
- Connecting Peptide (C-Peptide) CLIA: 2 X 50 Test 52025078Document2 pagesConnecting Peptide (C-Peptide) CLIA: 2 X 50 Test 52025078p11.sethiaNo ratings yet
- Appendix C 1 1Document12 pagesAppendix C 1 1MARIA VIORICANo ratings yet
- Proteina C ReactivaDocument2 pagesProteina C ReactivaMaria BlassNo ratings yet
- GUIDE TO IMMUNOHEMATOLOGY USING GEL TECHNOLOGYDocument92 pagesGUIDE TO IMMUNOHEMATOLOGY USING GEL TECHNOLOGYVivek PatelNo ratings yet
- AHG Anti IgG C3d PolyspecificDocument2 pagesAHG Anti IgG C3d PolyspecificEslam NassarNo ratings yet
- Maccura How To UseDocument2 pagesMaccura How To UserahmadyfeisalNo ratings yet
- Heat Elution - A Modification of The Landsteiner-Miller MethodDocument3 pagesHeat Elution - A Modification of The Landsteiner-Miller MethodCatia CorreaNo ratings yet
- Bovine BOVINE - SERUM - ALBUMINSerum AlbuminDocument3 pagesBovine BOVINE - SERUM - ALBUMINSerum AlbuminHaaelNo ratings yet
- Blood TypingDocument2 pagesBlood TypingDahr ELNo ratings yet
- Se 120112 BulDocument3 pagesSe 120112 Bul785Sachin Kumar KaushalNo ratings yet
- Detection & Id of AntibodiesDocument13 pagesDetection & Id of Antibodiessfjjq2pq2sNo ratings yet
- Antigen TypingDocument3 pagesAntigen Typingchristina.huntNo ratings yet
- Se 120151 BulDocument3 pagesSe 120151 BulAhmed AliNo ratings yet
- HSV 1 Igg: Elisa Test For The Detection of Igg Antibodies To Herpes Simplex Virus Type 1 in Human SerumDocument2 pagesHSV 1 Igg: Elisa Test For The Detection of Igg Antibodies To Herpes Simplex Virus Type 1 in Human SerumMaherNo ratings yet
- Mark Blood BankDocument20 pagesMark Blood BankMark Lester MoralesNo ratings yet
- GM.007 Crossmatch Anti IgG Gel CardDocument10 pagesGM.007 Crossmatch Anti IgG Gel CardKarl GutierrezNo ratings yet
- Fibrinogen 506 EngDocument6 pagesFibrinogen 506 Engالواثقة باللهNo ratings yet
- HSV 1-2 POOL IgM EI - 2531-1M - A - UK - C07Document12 pagesHSV 1-2 POOL IgM EI - 2531-1M - A - UK - C07muhammad febriadyNo ratings yet
- EUA Euroimmun ElisaG IfuDocument17 pagesEUA Euroimmun ElisaG IfuEdon BlakajNo ratings yet
- Nephchem ASODocument1 pageNephchem ASOsobujNo ratings yet
- Division of Blood Transfusion Services: Ministry of Health and Family WelfareDocument31 pagesDivision of Blood Transfusion Services: Ministry of Health and Family WelfareRaja SharmaNo ratings yet
- KBVH015-2 GENLISA Human Anti-Coronavirus Covid-19 IgG Spike Proteins Qualitative ELISA Ver2 0Document6 pagesKBVH015-2 GENLISA Human Anti-Coronavirus Covid-19 IgG Spike Proteins Qualitative ELISA Ver2 0KRISHGEN BIOSYSTEMSNo ratings yet
- Myoglobin Test System: Product Code: 3275-300Document2 pagesMyoglobin Test System: Product Code: 3275-300Jamil GonzasarNo ratings yet
- Blood Bank Laboratory Assignment 2 - Endterm Ahg TestDocument5 pagesBlood Bank Laboratory Assignment 2 - Endterm Ahg TestAnastasiaNo ratings yet
- HBSAG Rapid Test 2Document6 pagesHBSAG Rapid Test 2Charlotte OhNo ratings yet
- El ToxmDocument2 pagesEl ToxmMaherNo ratings yet
- SPEAKER: Dr. Subhajit Das MODERATOR: Prof. Jyoti ShuklaDocument25 pagesSPEAKER: Dr. Subhajit Das MODERATOR: Prof. Jyoti Shuklaswaraj sharma100% (2)
- Combs Test PRESENTATIONDocument20 pagesCombs Test PRESENTATIONaripoo.sufyan1No ratings yet
- Saline-Indirect Antiglobulin Test: Reagents/SuppliesDocument3 pagesSaline-Indirect Antiglobulin Test: Reagents/SuppliesCatia CorreaNo ratings yet
- BLOOD BANKING NOTES GUIDEDocument15 pagesBLOOD BANKING NOTES GUIDEThea Gonzales100% (3)
- Biology Module 5 - Animal Organ SystemsDocument34 pagesBiology Module 5 - Animal Organ SystemsfloNo ratings yet
- Circulatory SystemDocument5 pagesCirculatory SystemTan XiangNo ratings yet
- Medical Laboratory Tests: Tang PingDocument42 pagesMedical Laboratory Tests: Tang Pingapi-19916399No ratings yet
- Abo Blood Typing WsDocument3 pagesAbo Blood Typing Wsjanemarffy0% (2)
- ELS Q2 M6 Organ Systems of Representative Animals 1 RDocument22 pagesELS Q2 M6 Organ Systems of Representative Animals 1 RtjeremyalleneNo ratings yet
- Introduction To Physiological SystemsDocument10 pagesIntroduction To Physiological SystemsLim SuxingNo ratings yet
- Chapter1-The Clinical LabDocument24 pagesChapter1-The Clinical LabNawra AhmadNo ratings yet
- Osmosis 41Document1 pageOsmosis 41Sarawini KosolsombatNo ratings yet
- Shweta Jain (42Y/F) Aarogyam C: Report For Tests AskedDocument14 pagesShweta Jain (42Y/F) Aarogyam C: Report For Tests Askednit2000_jainNo ratings yet
- RPT SC Year 5 (DLP) 2022-2023Document24 pagesRPT SC Year 5 (DLP) 2022-2023norainippgsceNo ratings yet
- PallorDocument16 pagesPallorManal AlQuaimi100% (1)
- Reflective Type Blood Oxygen Saturation Detection System Based On MAX30100Document6 pagesReflective Type Blood Oxygen Saturation Detection System Based On MAX30100Emily JulianaNo ratings yet
- Kelimeler Sifatlar ORIGKEYDocument8 pagesKelimeler Sifatlar ORIGKEYErtuğrul YılmazNo ratings yet
- Short Note Biology Form 5-Chapter 1 TransportDocument7 pagesShort Note Biology Form 5-Chapter 1 Transportsalamah_sabri83% (6)
- Lecture 3. Bleeding Disorders Part 1Document31 pagesLecture 3. Bleeding Disorders Part 1Kekelwa Mutumwenu Snr100% (1)
- Elevated HBF Labelled As LA1CcHb1 On BioRad D10 HPLCDocument2 pagesElevated HBF Labelled As LA1CcHb1 On BioRad D10 HPLCsomething privateNo ratings yet
- Natural Anesthetics in The Transport of Nile Tilapia: Hematological and Biochemical Responses and Residual Concentration in The FilletDocument7 pagesNatural Anesthetics in The Transport of Nile Tilapia: Hematological and Biochemical Responses and Residual Concentration in The FilletSheila OliveiraNo ratings yet
- 2nd Day OF Exams in 1st QTR 2016Document9 pages2nd Day OF Exams in 1st QTR 2016John RoasaNo ratings yet
- PM Lividity ArticleDocument7 pagesPM Lividity ArticleatikaNo ratings yet
- Chapter One A CellDocument46 pagesChapter One A CellLeon MarkoNo ratings yet
- Chap8 PDFDocument81 pagesChap8 PDFJenny Acosta CatacutanNo ratings yet
- Homeostasis L1-4 Body SystemsDocument28 pagesHomeostasis L1-4 Body SystemsSK AuNo ratings yet
- Health Benefits of Geranium Essential OilDocument2 pagesHealth Benefits of Geranium Essential OilMolibeli LibetsoNo ratings yet
- Sherin Blancada GENERAL BIOLOGY Week1 6 4th Quarter ACAD STEM 1Document25 pagesSherin Blancada GENERAL BIOLOGY Week1 6 4th Quarter ACAD STEM 1Banana QNo ratings yet