Professional Documents
Culture Documents
2.2 Electron Configuration
Uploaded by
laura0 ratings0% found this document useful (0 votes)
32 views2 pagesIb chemistry 2.2
Original Title
2.2
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentIb chemistry 2.2
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
32 views2 pages2.2 Electron Configuration
Uploaded by
lauraIb chemistry 2.2
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
2.
2 Electron configuration
-
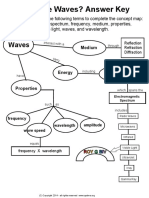
Electromagnetic spectrum shows all forms of radiation of differing energy, it
includes the visible light, which is the only part of the spectrum that we can see.
It ranges from the low energy radio waves to the high energy gamma rays, all of
the waves travel at the same speed, but are distinguishable by their wavelengths.
Different colours will have different wavelengths. Red has a higher wavelength
than violet light.
The EMS increases wavelength and frequency and decreases energy.
White light is a mixture of light waves of differing wavelengths or colours,
o This is seen when light passes through a prism and produces the different
colours. Producing a continuous spectrum.
When EM radiation passes through atoms they may absorb some of it, this will
excite their electrons from a lower energy level to a higher.
o This basically means that they jump from a lower energy level to a higher
one. Electrons cannot live within energy levels, so they simply appear.
(complicated quantum chemistry)
o When this happens they are said to be in an excited state, however this is
unstable and therefore the electron will soon fall back to the lowest level,
called the ground state.
o When this happens it gives out energy in the form of a packet known as a
photon, its energy is proportional to the frequency of the radiation it
absorbed.
o The energy of an emitted photon is equal to the energy change in an
atom.
An absorption light spectrum has all colours but some are missing (like dashes
are missing). This is produced when light passes through something like
hydrogen gas.
However, when energy is added (in the form of heat, electricity etc.), an emission
spectrum is produced. It only shows certain frequencies. They should be the
same that are missing from the absorption spectra.
o Full spectrum Absorption spectrum = emission spectrum
Different elements have different spectra; they may be used as barcodes to
identify each element. They also give us valuable info about their configuration.
Case study: HYDROGEN
o It produces visible light when an electron falls to n=2, when the electron
falls to n=1 it produces UV, it produces IR when it falls to n=3+.
o A big jump will give out lots of energy.
o Energy levels in an atom converge at high energies, because they get
closer together at higher energy.
o An electron at n= infinity ionises the atom, the energy needed to remove
one mole of gaseous atoms is called IONISATION ENERGY.
Uncertainty principle: we cant know for certain where an electron is at any given
moment, but we have a probability picture of where it might be.
o These regions are called ORBITALS, they are around an atomic nucleus
and theres a 90% probability of finding an electron there. When an
electron is in an orbital of higher energy it will have a higher probability of
being found further from the nucleus.
S orbital: sphere
P orbital: dumbbell3
You might also like
- SpectrosDocument15 pagesSpectrosNarmada BNo ratings yet
- Emission and Absorption SpectraDocument26 pagesEmission and Absorption SpectraRiriii67% (3)
- Electron Configurations-Transition, IonizationDocument33 pagesElectron Configurations-Transition, IonizationRayan BotanyNo ratings yet
- Spectral Lines: Existence of Discrete Energy Levels Emission and Absorption SpectraDocument69 pagesSpectral Lines: Existence of Discrete Energy Levels Emission and Absorption SpectraACSVNo ratings yet
- The Photoelectric Effect and Atomic Spectra: Dreanzel Corbin Pascua:P Mark John ReyesDocument41 pagesThe Photoelectric Effect and Atomic Spectra: Dreanzel Corbin Pascua:P Mark John ReyesRandy GasalaoNo ratings yet
- Report SpectrosDocument4 pagesReport SpectrosFff FffNo ratings yet
- Laser TherapyDocument8 pagesLaser Therapytagewub4No ratings yet
- Laser 1Document22 pagesLaser 1god.krish.25No ratings yet
- Unit 2 The Electronic Structure of Atoms and The Periodic TableDocument22 pagesUnit 2 The Electronic Structure of Atoms and The Periodic TableAragorn ChanNo ratings yet
- Line Spectra-G13Document7 pagesLine Spectra-G13Rushita LingiahNo ratings yet
- Laser LightDocument27 pagesLaser Lightdcool3784No ratings yet
- 4 DP Hydrogen SpectrumDocument21 pages4 DP Hydrogen SpectrumVaida MatulevičiūtėNo ratings yet
- The Development of A New Atomic ModelDocument4 pagesThe Development of A New Atomic ModelanaNo ratings yet
- Electronic StructureDocument26 pagesElectronic StructureDavidson ChanNo ratings yet
- LASERDocument30 pagesLASERanilm130484meNo ratings yet
- Atomic Structure Atomic Spectra and ElectronDocument11 pagesAtomic Structure Atomic Spectra and Electrontechibu252No ratings yet
- Spectroscopy: Analysis of Light Emitted or Absorbed by SubstancesDocument8 pagesSpectroscopy: Analysis of Light Emitted or Absorbed by SubstancesPearl LawrenceNo ratings yet
- AP Chemistry The Origin of Flame ColorsDocument2 pagesAP Chemistry The Origin of Flame ColorsNWong 63604No ratings yet
- Line SpectraDocument16 pagesLine SpectraYusuf AlamNo ratings yet
- Solar Cells: 1. TheoryDocument26 pagesSolar Cells: 1. TheoryStefan StanescuNo ratings yet
- Practical Work 7Document9 pagesPractical Work 7Achraf RabadiNo ratings yet
- Quantum PhysicsDocument67 pagesQuantum PhysicsxiaokiaNo ratings yet
- Emission and Absorption SpectraDocument2 pagesEmission and Absorption SpectraHossainNo ratings yet
- 2007 Electrons in AtomsDocument123 pages2007 Electrons in Atomsapi-293306937No ratings yet
- 2 3 Electron ArrangementDocument5 pages2 3 Electron ArrangementNguyenHoangMinhDucNo ratings yet
- Chap. 28 & 29: Photons, Quantum Physics & Structure of The AtomDocument4 pagesChap. 28 & 29: Photons, Quantum Physics & Structure of The AtomHaq NawazNo ratings yet
- Energy Levels & Photon Emission: Figure 1: Continuous Spectrum A. Emission SpectrumDocument6 pagesEnergy Levels & Photon Emission: Figure 1: Continuous Spectrum A. Emission SpectrumMahnoor SiddiqiNo ratings yet
- S.6 Atomic Structur Notes 1 Revision Past PapersDocument12 pagesS.6 Atomic Structur Notes 1 Revision Past PapersMaama PhionaNo ratings yet
- Description: Electromagnetic Radiation Charged ParticlesDocument8 pagesDescription: Electromagnetic Radiation Charged ParticlesfaithNo ratings yet
- BiophysicsDocument2 pagesBiophysicsKristina Casandra PavaNo ratings yet
- Unit V LasersDocument12 pagesUnit V LasersHrishi JohariNo ratings yet
- Quantitative and Qualitative Spectrophotometry: Dr. Ibrahim A. NaguibDocument75 pagesQuantitative and Qualitative Spectrophotometry: Dr. Ibrahim A. NaguibMoatsem AhmedNo ratings yet
- Laser System and Application: DirectionalityDocument19 pagesLaser System and Application: DirectionalityajityadavsNo ratings yet
- UV - Visible SpectrophotometryDocument171 pagesUV - Visible SpectrophotometryGudisa KufoNo ratings yet
- Topics: 26.4 Energy Levels in Atoms 26.5 Line Spectra: Ia2 Physics Cambridge A-Levels Teaching NotesDocument3 pagesTopics: 26.4 Energy Levels in Atoms 26.5 Line Spectra: Ia2 Physics Cambridge A-Levels Teaching NotesWahyu KurniawanNo ratings yet
- 4.5. Quantum PhysicsDocument4 pages4.5. Quantum PhysicsjmsonlNo ratings yet
- Chapter 5 Notes ShortXDocument46 pagesChapter 5 Notes ShortXdsckln/cmakNo ratings yet
- The Nature of Light: Does It Behave? These Questions May Seem Simple To You, But They Have PresentedDocument5 pagesThe Nature of Light: Does It Behave? These Questions May Seem Simple To You, But They Have PresentedAbdul QuadirNo ratings yet
- CHAPTER 2 Atomic Structure AND QUANTUM NUMBERDocument105 pagesCHAPTER 2 Atomic Structure AND QUANTUM NUMBERNurmaizatul AtikahNo ratings yet
- Line SpectrumDocument2 pagesLine Spectrum27yuvrajsoni10No ratings yet
- SL Chapter 2 ReviewDocument27 pagesSL Chapter 2 ReviewakikoNo ratings yet
- Chapter Two. EEE & ETI 1204 Material ScienceDocument29 pagesChapter Two. EEE & ETI 1204 Material ScienceKIRAGU PURITY NJERINo ratings yet
- AtomsStarLight 01Document18 pagesAtomsStarLight 01LukeMontanaNo ratings yet
- Spectroscopy: Electromagnetic RadiationDocument19 pagesSpectroscopy: Electromagnetic RadiationPriyanka SharmaNo ratings yet
- Laser Study MaterialDocument8 pagesLaser Study MaterialMohammed Rizwan MalikNo ratings yet
- Spectrometry FinalDocument57 pagesSpectrometry FinalAastha SahuNo ratings yet
- Laser TherapyDocument8 pagesLaser Therapyinrmpt77No ratings yet
- CYN002 Spectroscopy Introduction Class2 04042022 Send StudentsDocument24 pagesCYN002 Spectroscopy Introduction Class2 04042022 Send StudentsAYUSH KISHORENo ratings yet
- Unit 11 Laser and Its Medical Applications: 1. Summary About AtomsDocument16 pagesUnit 11 Laser and Its Medical Applications: 1. Summary About Atoms03152788No ratings yet
- Electronic Structure 3Document41 pagesElectronic Structure 3Victoria VirgoNo ratings yet
- L2 Che101Document16 pagesL2 Che101Musa Ahammed MahinNo ratings yet
- Interactions of Light and MatterDocument6 pagesInteractions of Light and MatterXin NiNo ratings yet
- 2nd Grading ScienceDocument6 pages2nd Grading ScienceFely CapangpanganNo ratings yet
- Atomic StructureDocument4 pagesAtomic StructureThea GermanNo ratings yet
- Electronic Structure of MatterDocument16 pagesElectronic Structure of MatterPrincess AnnNo ratings yet
- 1-Introduction To Spectrochemical MethodsDocument36 pages1-Introduction To Spectrochemical MethodsWahyuni EkaNo ratings yet
- Feynman Lectures Simplified 2A: Maxwell's Equations & ElectrostaticsFrom EverandFeynman Lectures Simplified 2A: Maxwell's Equations & ElectrostaticsNo ratings yet
- Lastexception 63666789675Document1 pageLastexception 63666789675lauraNo ratings yet
- Domingo Lunes Martes: Miss School?Document3 pagesDomingo Lunes Martes: Miss School?lauraNo ratings yet
- I B Maths Standard NotesDocument167 pagesI B Maths Standard NoteslauraNo ratings yet
- Lastexception 63666579063Document5 pagesLastexception 63666579063lauraNo ratings yet
- 4.1 Ionic Bonding and StructureDocument2 pages4.1 Ionic Bonding and StructurelauraNo ratings yet
- 5 Re-Evaluating Bowlby ArticleDocument6 pages5 Re-Evaluating Bowlby ArticlelauraNo ratings yet
- TOPIC1 Quantitative Q&ADocument25 pagesTOPIC1 Quantitative Q&AlauraNo ratings yet
- c1 Sequences ExamDocument2 pagesc1 Sequences ExamlauraNo ratings yet
- An Inspector CallsDocument17 pagesAn Inspector Callslaura0% (1)
- Chemistry Revision Pack - 2012Document20 pagesChemistry Revision Pack - 2012api-21735041071% (7)
- Chemistry Core Notes C1-10Document87 pagesChemistry Core Notes C1-10lauraNo ratings yet
- Cold WarDocument3 pagesCold WarlauraNo ratings yet
- 2.44 Homeostasis Cambridge IGCSEDocument15 pages2.44 Homeostasis Cambridge IGCSElauraNo ratings yet
- Some Notes For IGCSE BiologyDocument38 pagesSome Notes For IGCSE BiologylauraNo ratings yet
- Colour WheelDocument55 pagesColour WheelFathimath Shimtha100% (1)
- Lighting Calculation PDFDocument21 pagesLighting Calculation PDFebrande88% (8)
- Catalogue LED GBN - 2022 - T8CNDocument1 pageCatalogue LED GBN - 2022 - T8CNNguyễn Thành ChungNo ratings yet
- Utilization of Electrical Power: IlluminationDocument29 pagesUtilization of Electrical Power: IlluminationHafsa AlhaddabiNo ratings yet
- Kathrein 2017 Directional Antennas 4 Ports Dual Polarization 45°Document48 pagesKathrein 2017 Directional Antennas 4 Ports Dual Polarization 45°RobertNo ratings yet
- Santera Catalog Horoz Electrice 2010Document48 pagesSantera Catalog Horoz Electrice 2010Santera SRLNo ratings yet
- Circular Loop AntennaDocument23 pagesCircular Loop AntennaEC19B1042- Pendurthy Bhavana100% (1)
- Utv07 Utv-08Document1 pageUtv07 Utv-08Hector CardosoNo ratings yet
- Neonatal Phototherapy Unit: Keep This Manual For Future ReferenceDocument26 pagesNeonatal Phototherapy Unit: Keep This Manual For Future ReferenceDaniel TaiñaNo ratings yet
- Lesson Plan For Classroom ObservationDocument8 pagesLesson Plan For Classroom ObservationMichael FernandoNo ratings yet
- Traxon Catalogue 2018 (EU En)Document49 pagesTraxon Catalogue 2018 (EU En)fueledbyramenzNo ratings yet
- White LED DatasheetsDocument12 pagesWhite LED DatasheetsfaustogalvanNo ratings yet
- Blackbody Spectrum & Lasers SIM Homework Answer KeyDocument5 pagesBlackbody Spectrum & Lasers SIM Homework Answer KeyjerrenjgNo ratings yet
- Marco HID Lighting Catalog 12-81Document168 pagesMarco HID Lighting Catalog 12-81Alan MastersNo ratings yet
- LG PM9700Document7 pagesLG PM9700ty_at_cnetNo ratings yet
- EM Waves Concept MapDocument8 pagesEM Waves Concept MapAaron AsneNo ratings yet
- LED Vs Fluorescent - 10 Problems To Consider With Fluorescent LightingDocument11 pagesLED Vs Fluorescent - 10 Problems To Consider With Fluorescent LightingAnonymous KFOfMbNANo ratings yet
- Primary and Secondary ColorsDocument8 pagesPrimary and Secondary ColorskabadahijaNo ratings yet
- Atlas Lighting Lamp InformationDocument4 pagesAtlas Lighting Lamp InformationEliasNo ratings yet
- Fiber OpticsDocument26 pagesFiber OpticsCherry Ebreo OlaivarNo ratings yet
- Fish Tales Led Lamp Kit: PlayfieldDocument1 pageFish Tales Led Lamp Kit: PlayfieldDanijel CarNo ratings yet
- Eurolite TMH 150 PDFDocument2 pagesEurolite TMH 150 PDFKennethNo ratings yet
- Dewi Kurnia 60400118035 - Jurnal JFT Difraksi LaserDocument9 pagesDewi Kurnia 60400118035 - Jurnal JFT Difraksi LaserDewi KurniaNo ratings yet
- Mercury Vapor Lamp PDFDocument7 pagesMercury Vapor Lamp PDFaryaNo ratings yet
- BD - Bulk Erythrocyte Lysing With Ammonium Chloride For Flow Cytometry ImmunophenotypingDocument70 pagesBD - Bulk Erythrocyte Lysing With Ammonium Chloride For Flow Cytometry ImmunophenotypingBcells AutoimmunityNo ratings yet
- Fortimo LED Catalogue Feb 2014 19MB PDFDocument96 pagesFortimo LED Catalogue Feb 2014 19MB PDFglisha84No ratings yet
- 0 Microwave & Radar EngineeringDocument2 pages0 Microwave & Radar EngineeringChristy PollyNo ratings yet
- Unit 4 Polarization Q BankDocument2 pagesUnit 4 Polarization Q Bankfunkpatel123No ratings yet
- System HPLC - ClarkesDocument19 pagesSystem HPLC - ClarkesRulo RisculeseNo ratings yet
- Cores TDMDocument4 pagesCores TDMoriedagirbNo ratings yet