Professional Documents
Culture Documents
Chemguide - Answers: Electron Affinities
Uploaded by
S K MishraOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemguide - Answers: Electron Affinities
Uploaded by
S K MishraCopyright:
Available Formats
Chemguide answers
ELECTRON AFFINITIES
1. a) 142 kJ of energy is evolved when the change takes place.
b) The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an
electron to form 1 mole of gaseous 1- ions.
c) O(g) + e- O-(g)
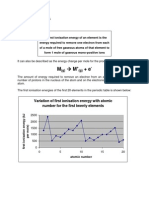
2. a) As you go down the group, the number of protons in the nucleus increases, for example from 17
in chlorine to 35 in bromine an increase of 18 protons. But the number of screening electrons
increases by exactly the same number from 10 inner electrons in chlorine to 28 inner electrons in
bromine.
That means that in all the elements in the group, an incoming electron feels a net pull from the
nucleus of 7+. But as you go down the group, the incoming electron ends up progressively further
from the nucleus, and so the attraction is less. A smaller attraction means that less energy will be
released.
b) This argument holds true for fluorine up to a point, but there is an additional factor you have to
think about. Fluorine is a small atom, and you are putting the new electron into an already electron-
rich space. This causes extra repulsions which cuts down the effect of the closeness of the electron
to the nucleus.
3. In both cases, the incoming electron is going into a 2p orbital, and so the screening and distance
from the nucleus is much the same for both atoms. However, oxygen has one fewer proton in the
nucleus, and so the attraction is less. A smaller attraction means that less energy will be released.
4. a) O-(g) + e- O2-(g)
b) The large positive value means that lots of energy has to be put in to force another electron into
the O- ion. This is because you are having to force a negative electron into a space which already
carries a negative charge.
c) In the oxygen case, the first electron to be added is going into a relatively small atom, and there is
significant repulsion from the electrons already there (similar to the fluorine case discussed above).
Sulphur is a bigger atom, and the effect of this repulsion is relatively less.
This effect also cuts down the amount of energy needed to force the second electron in to sulphur
relative to the amount needed for oxygen. The electrons are spread over a larger space in sulphur,
and so the repulsions aren't as great.
(Note: (b) and (c) overlap slightly. As long as you have covered all the points, it doesn't matter
particularly where exactly you have said them.)
www.chemguide.co.uk
You might also like
- Chemguide - Answers: Electron AffinitiesDocument1 pageChemguide - Answers: Electron AffinitiesDaneilla BanksNo ratings yet
- Patterns of First Ionisation Energies in The Periodic TableDocument7 pagesPatterns of First Ionisation Energies in The Periodic TableRugen RajNo ratings yet
- Classification of Elements Notes 3Document3 pagesClassification of Elements Notes 3Krishiv RajkumarNo ratings yet
- First Ionisation Energies (FIEsDocument6 pagesFirst Ionisation Energies (FIEsAathifa ThowfeekNo ratings yet
- CIE Chemistry A Level: 2: Atomic StructureDocument6 pagesCIE Chemistry A Level: 2: Atomic StructureahumanbeinginearthNo ratings yet
- CIE Chemistry A Level: 2: Atomic StructureDocument6 pagesCIE Chemistry A Level: 2: Atomic StructureMyraNo ratings yet
- IONISATION ENERGY PERIODIC TRENDDocument12 pagesIONISATION ENERGY PERIODIC TRENDhahajing1980No ratings yet
- 2 13 Ionisation EnergiesDocument6 pages2 13 Ionisation EnergiesRobertLiu100% (2)
- A - Level Inorganic Chemistry 2-1Document171 pagesA - Level Inorganic Chemistry 2-1kitderoger_391648570No ratings yet
- Electron Affinity: Is There A Pattern?Document4 pagesElectron Affinity: Is There A Pattern?Sabina SabaNo ratings yet
- Electronegativity ExplainedDocument2 pagesElectronegativity ExplainedDaneilla BanksNo ratings yet
- 3.1.1 Periodicity Workpack Answers v2 MBDocument11 pages3.1.1 Periodicity Workpack Answers v2 MBFRS gamingNo ratings yet
- Classification of ElementsDocument74 pagesClassification of ElementsJimit Patel BankNo ratings yet
- What is a Mass SpectrometerDocument11 pagesWhat is a Mass SpectrometerFiliz Kocayazgan ShanablehNo ratings yet
- Tanapp Tcm18-410hurDocument5 pagesTanapp Tcm18-410hurNguh FabriceNo ratings yet
- Inorganic Chemistry NotesDocument73 pagesInorganic Chemistry NotesYT ChongNo ratings yet
- Practice Problems Weeks 1 and 2Document2 pagesPractice Problems Weeks 1 and 2SandeshChapagainNo ratings yet
- Inorganic Chemistry NotesDocument105 pagesInorganic Chemistry NotesOdongo TonnyNo ratings yet
- Chem 111 Week Three Notes BDocument7 pagesChem 111 Week Three Notes BnyachiosirNo ratings yet
- Periodic Table and PeriodicityDocument11 pagesPeriodic Table and Periodicityakeemoluwadamilare623No ratings yet
- Atomic Radius and Effective Nuclear ChargeDocument8 pagesAtomic Radius and Effective Nuclear ChargeKisses EldswidthNo ratings yet
- Unit Objectives: Periodic TrendsDocument9 pagesUnit Objectives: Periodic Trendsmartin mulengaNo ratings yet
- A-LEVEL INORGANIC CHEMISTRY NOTESDocument55 pagesA-LEVEL INORGANIC CHEMISTRY NOTESaudrey abaasaNo ratings yet
- Periodicity Trends Across Periods 2 and 3Document7 pagesPeriodicity Trends Across Periods 2 and 3Anshu MovvaNo ratings yet
- Ion EnergiesDocument4 pagesIon EnergiesNamratha NairNo ratings yet
- Electron AffinityDocument51 pagesElectron AffinityS K MishraNo ratings yet
- Che Chapter 3Document7 pagesChe Chapter 3lisaNo ratings yet
- Homework Q - 10 MarkerDocument1 pageHomework Q - 10 Marker8ol78oNo ratings yet
- Bonding ChemistryDocument59 pagesBonding ChemistryKeshav CseNo ratings yet
- HL Electron Configuration and The Periodic TableDocument12 pagesHL Electron Configuration and The Periodic TableMarilee HuntNo ratings yet
- A Level Inorganic Chemistry NotesDocument95 pagesA Level Inorganic Chemistry NotesnaluwairoericjohnNo ratings yet
- Edexcel Chemistry Answers Combined FINALDocument50 pagesEdexcel Chemistry Answers Combined FINALboobla100% (2)
- Chem Inorganic A'levelDocument95 pagesChem Inorganic A'levelAlvin HavinzNo ratings yet
- As Chemistry 宝典 2023新版 Daisy-黑白5本 1Document80 pagesAs Chemistry 宝典 2023新版 Daisy-黑白5本 1bbcdgg1234567No ratings yet
- CHEMISTRY MCQs and AnswersDocument27 pagesCHEMISTRY MCQs and AnswersPen WomNo ratings yet
- Atomic Radii: Atomic Structure: Periodic TrendsDocument5 pagesAtomic Radii: Atomic Structure: Periodic TrendsRajat SharmaNo ratings yet
- A039level Chemistry Inorganic NotesDocument108 pagesA039level Chemistry Inorganic NotesNasser SsennogaNo ratings yet
- Chapter Eight BrownDocument13 pagesChapter Eight BrownJaka ShankalyanaNo ratings yet
- Chapter 10 Rate of ReactionDocument3 pagesChapter 10 Rate of ReactionLei YinNo ratings yet
- Chapter 3Document55 pagesChapter 3Imperial PlayzNo ratings yet
- First ionization energies explainedDocument1 pageFirst ionization energies explainedDaneilla BanksNo ratings yet
- Atomic Structure ElementsDocument39 pagesAtomic Structure ElementsAchini SheharaNo ratings yet
- Chemical Bonding ConceptsDocument91 pagesChemical Bonding ConceptsRomi IndayatiNo ratings yet
- Answer Key XI CH 3 Worksheet 2Document5 pagesAnswer Key XI CH 3 Worksheet 2iroonmaan123No ratings yet
- ChemistryDocument4 pagesChemistryAyah FNo ratings yet
- Ch30 Giancoli7e ManualDocument30 pagesCh30 Giancoli7e ManualRMNo ratings yet
- ChemistryDocument8 pagesChemistryJHALAK SINGHNo ratings yet
- Periodic Trends Atomic PropertiesDocument5 pagesPeriodic Trends Atomic PropertiesRizky KurniawatiNo ratings yet
- Ionization Energy and Electron AffinityDocument9 pagesIonization Energy and Electron AffinityKhan AaghaNo ratings yet
- Element Formula Systematic Name Common Name Element Formula Systematic Name Common NameDocument2 pagesElement Formula Systematic Name Common Name Element Formula Systematic Name Common NameBittuNo ratings yet
- Answer Key Term-1 Chemistry Class XI (2022-23)Document10 pagesAnswer Key Term-1 Chemistry Class XI (2022-23)Sumit RautNo ratings yet
- Periodic Properties - Part 3Document38 pagesPeriodic Properties - Part 3Bhavesh GargNo ratings yet
- AS Edexcel Chemistry Unit 1 Revision NotesDocument5 pagesAS Edexcel Chemistry Unit 1 Revision NotesTheMagicCarpetNo ratings yet
- Atomic structure, bonding and molesDocument22 pagesAtomic structure, bonding and molesJoshuaCookNo ratings yet
- 4th ClassDocument11 pages4th Classvaibhav baluNo ratings yet
- Atomic Structure, Bonding and Mass SpectrometryDocument7 pagesAtomic Structure, Bonding and Mass SpectrometryHumoon AfsardeirNo ratings yet
- Electrons in Atoms.Document62 pagesElectrons in Atoms.Md.Tanjim reza TurjoNo ratings yet
- Ionisation Energy ClassDocument13 pagesIonisation Energy ClassKordell McShineNo ratings yet
- Autophagy NobelDocument5 pagesAutophagy Nobelvenkatesh venkyNo ratings yet
- Lecture 29Document9 pagesLecture 29S K MishraNo ratings yet
- Fortunate Year of LifeDocument2 pagesFortunate Year of LifeS K MishraNo ratings yet
- Modi Sarkar 2.0: Portfolio AllocationDocument6 pagesModi Sarkar 2.0: Portfolio AllocationSurabhi PandeyNo ratings yet
- Lecture 30Document11 pagesLecture 30S K MishraNo ratings yet
- Bandhana Yoga, Arrest, Jail or ImprisonmentDocument3 pagesBandhana Yoga, Arrest, Jail or ImprisonmentS K Mishra100% (1)
- The Karmic MoonDocument4 pagesThe Karmic MoonS K Mishra100% (1)
- Result CardDocument52 pagesResult CardfoziaNo ratings yet
- Quantitative Analysis by ColorimetryDocument3 pagesQuantitative Analysis by ColorimetryS K Mishra100% (1)
- Lecture 27 PDFDocument11 pagesLecture 27 PDFS K MishraNo ratings yet
- Vipassana Q ADocument7 pagesVipassana Q AS K MishraNo ratings yet
- Lecture 31Document9 pagesLecture 31S K MishraNo ratings yet
- Beeja SphutaDocument5 pagesBeeja SphutaS K MishraNo ratings yet
- HR Shankar's EquationDocument1 pageHR Shankar's EquationS K Mishra100% (1)
- Lecture 28 PDFDocument10 pagesLecture 28 PDFS K MishraNo ratings yet
- Lecture 29 PDFDocument9 pagesLecture 29 PDFS K MishraNo ratings yet
- Lecture 36 - Group 18 (8A) : 2P32 - Inorganic ChemistryDocument8 pagesLecture 36 - Group 18 (8A) : 2P32 - Inorganic ChemistryS K MishraNo ratings yet
- Lecture 32 - Group 17: 2P32 - Principles of Inorganic Chemistry Dr.M.PilkingtonDocument18 pagesLecture 32 - Group 17: 2P32 - Principles of Inorganic Chemistry Dr.M.PilkingtonS K MishraNo ratings yet
- Lecture 35 - Group 17: 2P32 - Inorganic ChemistryDocument9 pagesLecture 35 - Group 17: 2P32 - Inorganic ChemistryS K MishraNo ratings yet
- Professor Melanie PilkingtonDocument2 pagesProfessor Melanie PilkingtonS K MishraNo ratings yet
- Lecture 30 PDFDocument11 pagesLecture 30 PDFS K MishraNo ratings yet
- Lecture 33 - Group 18 (8A) : 2P32 - Principles of Inorganic Chemistry Dr. M. PilkingtonDocument16 pagesLecture 33 - Group 18 (8A) : 2P32 - Principles of Inorganic Chemistry Dr. M. PilkingtonS K MishraNo ratings yet
- Lecture 5 PDFDocument10 pagesLecture 5 PDFS K MishraNo ratings yet
- Lecture 4 PDFDocument9 pagesLecture 4 PDFS K MishraNo ratings yet
- Lecture 1 - Recapping Important Concepts: 2P32 Winter Term 2012-13 Principles of Inorganic Chemistry Dr. M. PilkingtonDocument13 pagesLecture 1 - Recapping Important Concepts: 2P32 Winter Term 2012-13 Principles of Inorganic Chemistry Dr. M. PilkingtonS K Mishra0% (1)
- Lecture 4Document9 pagesLecture 4S K MishraNo ratings yet
- Lecture 3Document11 pagesLecture 3S K MishraNo ratings yet
- Periodic properties trendsDocument10 pagesPeriodic properties trendsS K MishraNo ratings yet
- 06Document11 pages06S K Mishra100% (1)
- Introduction To Oracle GroovyDocument53 pagesIntroduction To Oracle GroovyDeepak BhagatNo ratings yet
- Training Matrix For TM IDocument14 pagesTraining Matrix For TM IApril NavaretteNo ratings yet
- Tips and Tricks I: Getting the Most Out of ArcGIS DesktopDocument108 pagesTips and Tricks I: Getting the Most Out of ArcGIS Desktoptanja222No ratings yet
- Microstation V8I Accudraw Basics: Bentley Institute Course GuideDocument80 pagesMicrostation V8I Accudraw Basics: Bentley Institute Course Guideh_eijy2743No ratings yet
- Excel Dynamic Arrays: Department Item Quantity Price Total $Document5 pagesExcel Dynamic Arrays: Department Item Quantity Price Total $Bilal Hussein SousNo ratings yet
- General 04 Fixed Flow Pump To Three TanksDocument13 pagesGeneral 04 Fixed Flow Pump To Three TanksjpalauguillemNo ratings yet
- Module 1 Introduction To Highway and Railroad EngineeringDocument43 pagesModule 1 Introduction To Highway and Railroad EngineeringKenneth FajardoNo ratings yet
- M6 2020 Binomial Distribution Lecture NotesDocument27 pagesM6 2020 Binomial Distribution Lecture Notescoyite8695No ratings yet
- VSD Operacion ControlDocument138 pagesVSD Operacion ControlLeon PerezNo ratings yet
- Astm A6 A6m-08Document62 pagesAstm A6 A6m-08Vũ Nhân HòaNo ratings yet
- DbintfcDocument80 pagesDbintfchnr.uninstallNo ratings yet
- LyonDCCT Technology ReviewDocument4 pagesLyonDCCT Technology Reviewrajagopal gNo ratings yet
- Tradesman Electronics PDFDocument13 pagesTradesman Electronics PDFsandeepxrNo ratings yet
- Massive MIMO For Communications With Drone SwarmsDocument26 pagesMassive MIMO For Communications With Drone SwarmsAsher Suranjith JayakumarNo ratings yet
- Qualcomm LTE Performance & Challenges 09-01-2011Document29 pagesQualcomm LTE Performance & Challenges 09-01-2011vembri2178100% (1)
- Climate Change: The Fork at The End of NowDocument28 pagesClimate Change: The Fork at The End of NowMomentum Press100% (1)
- A320 CBT Test 1 PDFDocument107 pagesA320 CBT Test 1 PDFCesarNo ratings yet
- Low-Complexity Iterative Detection For Large-Scale Multiuser MIMO-OFDM Systems Using Approximate Message PassingDocument14 pagesLow-Complexity Iterative Detection For Large-Scale Multiuser MIMO-OFDM Systems Using Approximate Message PassingNitin KumarNo ratings yet
- SDH TechnologyDocument26 pagesSDH TechnologyJayesh SinghalNo ratings yet
- Classification of Differential Equations For Finding Their SolutionsDocument2 pagesClassification of Differential Equations For Finding Their SolutionsakhileshNo ratings yet
- Sensors 22 09378 v2Document13 pagesSensors 22 09378 v2FahdNo ratings yet
- Product - 20V4000G24F 3B FODocument32 pagesProduct - 20V4000G24F 3B FOmohammed khadrNo ratings yet
- Reliability EngineeringDocument9 pagesReliability Engineeringnvaradharajan1971No ratings yet
- Power Cable Installation ManualDocument50 pagesPower Cable Installation ManualAnn DodsonNo ratings yet
- Water Reducing - Retarding AdmixturesDocument17 pagesWater Reducing - Retarding AdmixturesAbdullah PathanNo ratings yet
- Haidarali-MR-2011-PhD-Thesis 01 PDFDocument378 pagesHaidarali-MR-2011-PhD-Thesis 01 PDFIbrahim KhanNo ratings yet
- Impeller: REV Rev by Description PCN / Ecn Date CHK'D A JMM Released For Production N/A 18/11/2019 PDLDocument1 pageImpeller: REV Rev by Description PCN / Ecn Date CHK'D A JMM Released For Production N/A 18/11/2019 PDLSenthilkumar RamalingamNo ratings yet
- UnderstandingCryptology CoreConcepts 6-2-2013Document128 pagesUnderstandingCryptology CoreConcepts 6-2-2013zenzei_No ratings yet
- PDS OperatorStationDocument7 pagesPDS OperatorStationMisael Castillo CamachoNo ratings yet
- Analisis Pengaruh Profitabilitas, Strategi Diversifikasi, Dan Good Corporate Governance Terhadap Nilai PerusahaanDocument16 pagesAnalisis Pengaruh Profitabilitas, Strategi Diversifikasi, Dan Good Corporate Governance Terhadap Nilai PerusahaanEra ZsannabelaNo ratings yet