Professional Documents
Culture Documents
Sodium
Uploaded by
mechfree0 ratings0% found this document useful (0 votes)
2 views31 pagesSodium
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentSodium
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
2 views31 pagesSodium
Uploaded by
mechfreeSodium
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 31
2resiz0re
WIKIPEDIA
Sodium - Wikipedia
Sodium
Intpsuifen wikipedia orgiwikiSodium
Sodium, ,,Na
Appearance silvery white metallic
Standard atomic 22.989 769 28(2)"]
weight (A,, standard)
7 Li
tT
Na
L
Hl K
neon — sodium — magnesium
Atomic number (Z) ah
Group group 1 (alkali metals)
Period period 3
Element category B alkali metal
Block s-block
Electron configuration [Ne] 3s‘
st
2resiz0re
hitpsfen wikipedia orgiwkilSodium
Sodium - Wikipedia
Electrons per shell
2,8,1
Phase at STP
Melting point
Boiling point
Density (near rt.)
when liquid (at m,
Critical point
Heat of fusion
Heat of vaporization
Molar heat capacity
solid
370.944 K (97.794 °C,
208.029 °F)
1156.090 K (882.940 °C,
1621.292 °F)
0.968 g/cm?
0.927 g/cm?
2573 K, 35 MPa
(extrapolated)
2.60 kJ/mol
97.42 kJ/mol
28.230 J/(mol-K)
Vapor pressure
P(Pa) | 4 | 10
at I (K)
554 | 617 697 | 802 | 946
100 | 1k | 10k 100k
1153
Oxidation states
Electronegativity
lonization energies
Atomic radius
Covalent radius
+1, -1 (a strongly basic
oxide)
Pauling scale: 0.93
Ast: 495.8 kJ/mol
2nd: 4562 kJ/mol
3rd: 6910.3 kJ/mol
(more)
empirical: 186 pm
16649 pm
2181
hitpsfen wikipedia orgiwkilSodium
Sodium - Wikipedia
Van der Waals radius
227 pm
Spectral lines
Crystal structure
Speed of sound
thin rod
Thermal expansion
Thermal conductivity
Electrical resistivity
Magnetic ordering
Magnetic
suscepti
ity
Young's modulus
Shear modulus
Bulk modulus
Mohs hardness
Brinell hardness
CAS Number
body-centered cubic
-
3200 m/s (at 20 °C)
71 ym/(m-kK) (at 25 °C)
142 Wi(m-k)
47.7 nQ-m (at 20 °C)
paramagnetic!2]
+16.0:10°6 cm3/mol
(298 kK)
10 GPa
3.3 GPa
6.3 GPa
0.5
0.69 MPa
7440-23-5
Discovery and first
isolation
Humphry Davy (1807)
Abun-
dance
Iso-
tope
Half-life
(tia)
Pro-
duct
Decay
mode
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- 2222Document1 page2222mechfreeNo ratings yet
- 7 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page7 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- Logitech MX Vertical DatasheetDocument2 pagesLogitech MX Vertical DatasheetmechfreeNo ratings yet
- 21Document1 page21mechfreeNo ratings yet
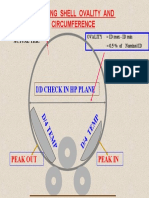
- Checking Shell Ovality and Circumference: I/D Check in HP PlaneDocument1 pageChecking Shell Ovality and Circumference: I/D Check in HP PlanemechfreeNo ratings yet
- Templates: To Check The Inside Diameter Made Out of 3-4Mm Thickness Plates D/4 To D/8 TemplatesDocument1 pageTemplates: To Check The Inside Diameter Made Out of 3-4Mm Thickness Plates D/4 To D/8 TemplatesmechfreeNo ratings yet
- 5 - PDFsam - Designing Fillet Welds For Skewed T-Jointsâ - Part 1 - The James F ...Document1 page5 - PDFsam - Designing Fillet Welds For Skewed T-Jointsâ - Part 1 - The James F ...mechfreeNo ratings yet
- 1 - PDFsam - Designing Fillet Welds For Skewed T-Jointsâ - Part 1 - The James F ...Document1 page1 - PDFsam - Designing Fillet Welds For Skewed T-Jointsâ - Part 1 - The James F ...mechfreeNo ratings yet
- 4 - PDFsam - Designing Fillet Welds For Skewed T-Jointsâ - Part 1 - The James F ...Document1 page4 - PDFsam - Designing Fillet Welds For Skewed T-Jointsâ - Part 1 - The James F ...mechfreeNo ratings yet
- 10 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page10 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- 3 - PDFsam - Designing Fillet Welds For Skewed T-Jointsâ - Part 1 - The James F ...Document1 page3 - PDFsam - Designing Fillet Welds For Skewed T-Jointsâ - Part 1 - The James F ...mechfreeNo ratings yet
- Good Engineering Practices (For Rolling)Document1 pageGood Engineering Practices (For Rolling)mechfreeNo ratings yet
- Welding Code, As Well As Other AWS Publications (I.e., The Welding Handbook, Ninth Edition, Volume 1) - Such TermiDocument1 pageWelding Code, As Well As Other AWS Publications (I.e., The Welding Handbook, Ninth Edition, Volume 1) - Such TermimechfreeNo ratings yet
- 14 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page14 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- Peak in / Peak Out: Over / Under Bending During Edge Breaking OperationDocument1 pagePeak in / Peak Out: Over / Under Bending During Edge Breaking OperationmechfreeNo ratings yet
- 21 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page21 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- 20 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page20 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- 18 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page18 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- Tolerances On Circ. of Shells, D'Ends, Cones & BellowsDocument1 pageTolerances On Circ. of Shells, D'Ends, Cones & BellowsmechfreeNo ratings yet
- 12 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page12 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- Procedure For Marking, Cutting and Checking of Shell PlatesDocument1 pageProcedure For Marking, Cutting and Checking of Shell PlatesmechfreeNo ratings yet
- 6 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page6 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- Rolling Depends: Provide Necessary Allowance (1.5 To 2 Times The Thickness)Document1 pageRolling Depends: Provide Necessary Allowance (1.5 To 2 Times The Thickness)mechfreeNo ratings yet
- Good Engineering Practices (For Rolling)Document1 pageGood Engineering Practices (For Rolling)mechfreeNo ratings yet
- 13 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page13 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- 19 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page19 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- 8 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page8 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- Cold Rolling: Rolling at Normal Room TemperatureDocument1 pageCold Rolling: Rolling at Normal Room TemperaturemechfreeNo ratings yet
- 22 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page22 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet
- 9 PDFsam Shellrollingprocedure 131019050223 Phpapp01Document1 page9 PDFsam Shellrollingprocedure 131019050223 Phpapp01mechfreeNo ratings yet