Professional Documents
Culture Documents
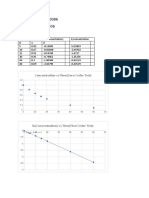
Plot of Langmuir Isotherm
Uploaded by
Maharghya BiswasOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Plot of Langmuir Isotherm
Uploaded by
Maharghya BiswasCopyright:
Available Formats
Ci Ce qe 1/Ce 1/qe logCe logqe
151 22 32.25 0.045455 0.031008 1.342423 1.50853
199 56.5 35.625 0.017699 0.02807 1.752048 1.551755
245 95 37.5 0.010526 0.026667 1.977724 1.574031
300 150 37.5 0.006667 0.026667 2.176091 1.574031
353 200 38.25 0.005 0.026144 2.30103 1.582631
400 246 38.5 0.004065 0.025974 2.390935 1.585461
446 290 39 0.003448 0.025641 2.462398 1.591065
493 340 38.25 0.002941 0.026144 2.531479 1.582631
Plot of Langmuir Isotherm
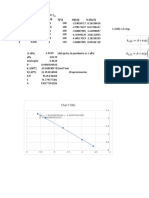
0.035
0.03
0.025
0.02
1/qe
y = 0.1214x + 0.0256
0.015
R² = 0.9807
0.01
0.005
0
0 0.01 0.02 0.03 0.04 0.05
1/Ce
For Langmuir Isotherm, 1/qe = (1/qm* KL)*Ce + 1/qm
So, 1/qm = 0.0256 (Intercept)
Therefore, qm = 39.0625
(1/qm*KL) =0.1214 so, KL = 0.21
Therefore, the value of Langmuir Isotherm constant, KL = 0.21
Plot of Freundlisch Isotherm
1.6
y = 0.0632x + 1.4351
1.59 R² = 0.8984
1.58
1.57
1.56
logqe
1.55
1.54
1.53
1.52
1.51
1.5
0 1 logCe 2 3
For Freundlich Isotherm, LOG (qe) = (1/n)* LOG (Ce) + LOG (KF)
So, LOG (KF) = 1.4351 (Intercept) and (1/n) = 0.0632 so, n=15.822
Therefore, KF = 10^(1.4351) = 27.233
So, the value of Freundlich Isotherm constant, KF = 27.233
For langmuir isotherm,
the regression coefficient value is 0.9807
which is more closer to 1
while regression coefficient
for Freundlisch isotherm is 0.8984.
Hence, langmuir isotherm is more
appropriate for the given set
of values of Ci and Ce.
You might also like
- Langmuir Isotherm Model Fits Adsorption Data Better Than FreundlichDocument2 pagesLangmuir Isotherm Model Fits Adsorption Data Better Than FreundlichMaharghya BiswasNo ratings yet
- Langmuir Isotherm Model for Adsorption DataDocument2 pagesLangmuir Isotherm Model for Adsorption DataMaharghya BiswasNo ratings yet
- Langmuir Isotherm Model for Adsorption DataDocument2 pagesLangmuir Isotherm Model for Adsorption DataMaharghya BiswasNo ratings yet
- Langmuir and Freundlich Isotherm Analysis of Adsorption DataDocument2 pagesLangmuir and Freundlich Isotherm Analysis of Adsorption DataMaharghya BiswasNo ratings yet
- C PhiDocument5 pagesC PhiTiếnVũNo ratings yet
- Nabila Kartika Yumna 195070301111001 A1 25 LapPraktikumKadaluarsaPangan.Document5 pagesNabila Kartika Yumna 195070301111001 A1 25 LapPraktikumKadaluarsaPangan.nabila kartikaNo ratings yet
- Benito Cano Rossmel - Ejercicios ResueltoDocument10 pagesBenito Cano Rossmel - Ejercicios ResueltoCristian Rosmel Benito CanoNo ratings yet
- Ammonia Design 2520of 2520equipmentsDocument32 pagesAmmonia Design 2520of 2520equipmentsapi-3714811100% (1)
- Where P : A. Flowrate Air Merubah SCFM Menjadi Lbmol/minDocument4 pagesWhere P : A. Flowrate Air Merubah SCFM Menjadi Lbmol/minCarissa EilinNo ratings yet
- Egyptian Code 2008 earthquake forces calculatorDocument11 pagesEgyptian Code 2008 earthquake forces calculatorkhaled nasrNo ratings yet
- Tarun Tripathy - 200386 Vansh Hooda - 200336 Ans 1-: Concentration Vs Time (Zero Order Test)Document3 pagesTarun Tripathy - 200386 Vansh Hooda - 200336 Ans 1-: Concentration Vs Time (Zero Order Test)Kriti TripathyNo ratings yet
- IsotermasDocument14 pagesIsotermasDaniella HidalgoNo ratings yet
- Ministry of Higher Education and Scientific Research: Kirkuk Civil Engineering Stage - ThreeDocument8 pagesMinistry of Higher Education and Scientific Research: Kirkuk Civil Engineering Stage - Threeali najatNo ratings yet
- Ejercicio 3Document2 pagesEjercicio 3Jose David RodriguezNo ratings yet
- Densidad de MurosDocument7 pagesDensidad de MurosGRUPOKNo ratings yet
- Azucares ReductoresDocument8 pagesAzucares ReductoresYorleidys AvilezNo ratings yet
- BATCH SETTLING OF SOLID SLURRIES: MOTIVATION, OBJECTIVES, THEORY, EXPERIMENTAL PROCEDURE AND CALCULATIONDocument25 pagesBATCH SETTLING OF SOLID SLURRIES: MOTIVATION, OBJECTIVES, THEORY, EXPERIMENTAL PROCEDURE AND CALCULATIONSUYASH SUNIL ABNAVE PGP 2020 BatchNo ratings yet
- Jurnal Wei Berdasarkan Tabel 1 Tentang Karakteristik BatubaraDocument17 pagesJurnal Wei Berdasarkan Tabel 1 Tentang Karakteristik BatubaraSii MettaNo ratings yet
- GraficasDocument3 pagesGraficasMirna Quistian ZarazuaNo ratings yet
- Earthquake EgyptDocument13 pagesEarthquake EgyptMostafa SeyamNo ratings yet
- Datos Exp Practica 2Document2 pagesDatos Exp Practica 2AsileUGarciaNo ratings yet
- Ca (Campuran) Konduktivitas 0.005 0.45 0.01 0.85 0.015 1.19 0.02 1.68 0.025 1.91Document3 pagesCa (Campuran) Konduktivitas 0.005 0.45 0.01 0.85 0.015 1.19 0.02 1.68 0.025 1.91MuhammadAmanNo ratings yet
- Initial Concentrati On, C Half-Life, T (Gmol L) : Chart TitleDocument4 pagesInitial Concentrati On, C Half-Life, T (Gmol L) : Chart TitleCamila TorresNo ratings yet
- Experiment 7Document5 pagesExperiment 7Tyler PhillipsNo ratings yet
- AROMA SEBELUM DISEDUH ORDO 0 DAN 1Document7 pagesAROMA SEBELUM DISEDUH ORDO 0 DAN 1Ni'mah Izati Atiko PutriNo ratings yet
- DataDocument18 pagesDataMohaiminNo ratings yet
- Pharmacokinetic analysis of drug absorption and eliminationDocument3 pagesPharmacokinetic analysis of drug absorption and eliminationFilzah Falvika PutriNo ratings yet
- Xi Yi/KiDocument12 pagesXi Yi/Kilevin sanchez gongoraNo ratings yet
- Problem A RioDocument31 pagesProblem A RioJose MiguelNo ratings yet
- Kellogg Synthesis Reactor Properties AnalysisDocument9 pagesKellogg Synthesis Reactor Properties AnalysisMainul HaqueNo ratings yet
- 2.5 Eadie (1942) Measured The Initial Reaction Rate of Hydrolysis of Acetylcholme (Substrate) byDocument6 pages2.5 Eadie (1942) Measured The Initial Reaction Rate of Hydrolysis of Acetylcholme (Substrate) byRoxan Bueno MoraNo ratings yet
- Test ModelDocument17 pagesTest ModelThanapat ThepubonNo ratings yet
- Kadar Absorbansi vs WaktuDocument2 pagesKadar Absorbansi vs WaktuReynand ThoriqNo ratings yet
- Kadar Vs Absorbansi: 0.8 1 F (X) 0.17867910543131 X + 0.011653418530352 R 0.997533821926732Document2 pagesKadar Vs Absorbansi: 0.8 1 F (X) 0.17867910543131 X + 0.011653418530352 R 0.997533821926732Reynand ThoriqNo ratings yet
- Batch Reactor CPC FinalDocument56 pagesBatch Reactor CPC FinalRUTUJA PINGALENo ratings yet
- Prilling Tower Desing FinalDocument5 pagesPrilling Tower Desing FinalCHUCHUNo ratings yet
- Gas Dynamics LabDocument4 pagesGas Dynamics Labomar atefNo ratings yet
- International Lateral LoadsDocument38 pagesInternational Lateral Loadshabibur Rahman KhanNo ratings yet
- Optimized Levenspiel Plot AnalysisDocument14 pagesOptimized Levenspiel Plot AnalysisAqib LatifNo ratings yet
- FluidosDocument4 pagesFluidosJavier Ramirez DiazNo ratings yet
- Luas frame filter press analysisDocument5 pagesLuas frame filter press analysisratih widianaNo ratings yet
- Well Discharge TestDocument8 pagesWell Discharge Testamy ackerNo ratings yet
- V= 2100 L 4.25 mol/L 2.5 mol/L flujo= 335 L/min Cp= 3.55 292 K ρ= 1100 ∆H= -53000 J/mol τ= 6.26865672 (min) 0.58823529 Ca = Cb = �+�→�Document6 pagesV= 2100 L 4.25 mol/L 2.5 mol/L flujo= 335 L/min Cp= 3.55 292 K ρ= 1100 ∆H= -53000 J/mol τ= 6.26865672 (min) 0.58823529 Ca = Cb = �+�→�Ana HernandezNo ratings yet
- BiochemprintfinalDocument14 pagesBiochemprintfinalJomhel CalluengNo ratings yet
- Practica MRUADocument3 pagesPractica MRUAMr. Hot RodNo ratings yet
- HBBVDocument5 pagesHBBVA.zhalzhabilla azzahrah AzzahrahNo ratings yet
- Hubungan Porositas Dan K: Nama: Dwisthi Satiti NIM: 113160126 Kelas: E (Metode Numerik)Document2 pagesHubungan Porositas Dan K: Nama: Dwisthi Satiti NIM: 113160126 Kelas: E (Metode Numerik)Dwisthi SatitiNo ratings yet
- Strain Gage GeneralDocument24 pagesStrain Gage Generalfech984No ratings yet
- CTM/CFPM FN 4/CfpmDocument2 pagesCTM/CFPM FN 4/CfpmKuo1018No ratings yet
- Design of packed absorption columnDocument29 pagesDesign of packed absorption columnJu Naid MalikNo ratings yet
- Properties of Seawater (35 charactersDocument2 pagesProperties of Seawater (35 charactersHartinie MNo ratings yet
- Solución de Box Culvert Por CDocument53 pagesSolución de Box Culvert Por Cjorge torres villalobosNo ratings yet
- Examen Resuelto FTM 2P 2018Document30 pagesExamen Resuelto FTM 2P 2018B Deni R RomeroNo ratings yet
- Datos T 20 Min T 220 F No 1.00E+07 Esp/Ml K 0.01 KT 0.2 LN No 16.1180957 Lnno - KT 15.9180957 MinDocument12 pagesDatos T 20 Min T 220 F No 1.00E+07 Esp/Ml K 0.01 KT 0.2 LN No 16.1180957 Lnno - KT 15.9180957 MinLuis TejedaNo ratings yet
- International Lateral LoadsDocument37 pagesInternational Lateral LoadsNELSONHUGONo ratings yet
- Albañileria Hoja de CalculoDocument6 pagesAlbañileria Hoja de CalculoE Altamirano PardoNo ratings yet
- UntitledDocument8 pagesUntitledLucas Hernández Karla BereniceNo ratings yet
- Design of Absorption Column 160127152306Document33 pagesDesign of Absorption Column 160127152306Dũng LêNo ratings yet
- 0314053120P101050806 Tarun Kumar PodderDocument3 pages0314053120P101050806 Tarun Kumar PodderMaharghya BiswasNo ratings yet
- 0314053120P101251625 Basudeb PolicyDocument2 pages0314053120P101251625 Basudeb PolicyMaharghya BiswasNo ratings yet
- United India Insurance Private Car Insurance CertificateDocument2 pagesUnited India Insurance Private Car Insurance CertificateMaharghya BiswasNo ratings yet
- Coal and Petroleum Multiple Choice QuestionsDocument6 pagesCoal and Petroleum Multiple Choice QuestionsMaharghya BiswasNo ratings yet
- Class10 Math Appendix2 NCERT TextBook EnglishEditionDocument11 pagesClass10 Math Appendix2 NCERT TextBook EnglishEditiongopal786No ratings yet
- Math 10Document32 pagesMath 10ShailendraPatelNo ratings yet
- 02 MatsuiDocument61 pages02 MatsuiuttamksrNo ratings yet
- Appendix 1Document21 pagesAppendix 1Saravanan AnnamalaiNo ratings yet
- Block1 506Document47 pagesBlock1 506Mitindra KonjengbamNo ratings yet
- Sulfuric Acid Material BalanceDocument9 pagesSulfuric Acid Material BalanceBernie_Garcia__9886100% (2)
- Windows 8 - Notice PDFDocument1 pageWindows 8 - Notice PDFSanthosh KumarNo ratings yet
- Block1 510Document122 pagesBlock1 510Maharghya BiswasNo ratings yet
- Final Project Aai FinalDocument63 pagesFinal Project Aai Finalxyz100% (11)
- Hesc105 PDFDocument8 pagesHesc105 PDFTop Treding UpdatesNo ratings yet
- Block1 508Document130 pagesBlock1 508dWADWNo ratings yet
- Unit 1 Nature of Social Sciences: StructureDocument54 pagesUnit 1 Nature of Social Sciences: StructureMaharghya BiswasNo ratings yet
- Sensors and Actuators B: ChemicalDocument7 pagesSensors and Actuators B: ChemicalMaharghya BiswasNo ratings yet
- Accepted Manuscript: Applied Surface ScienceDocument20 pagesAccepted Manuscript: Applied Surface ScienceMaharghya BiswasNo ratings yet
- Importance of Learning Mathematics at Elementary LevelDocument87 pagesImportance of Learning Mathematics at Elementary LevelMaharghya BiswasNo ratings yet
- Block1 505Document78 pagesBlock1 505Maharghya BiswasNo ratings yet
- 03 31.1.2018Document22 pages03 31.1.2018Maharghya BiswasNo ratings yet
- Block1 502Document127 pagesBlock1 502Maharghya BiswasNo ratings yet
- Block1 501Document87 pagesBlock1 501Maharghya BiswasNo ratings yet
- Society and EducationDocument99 pagesSociety and EducationJosé Alejandro Pérez RamírezNo ratings yet
- Learning Languages at Elementary Level: Course-503Document69 pagesLearning Languages at Elementary Level: Course-503Maharghya BiswasNo ratings yet
- Aspen Question Maharghya and SayaniDocument2 pagesAspen Question Maharghya and SayaniMaharghya BiswasNo ratings yet
- Atmospheric Pollution Research: Iman Parseh, Hakimeh Teiri, Yaghoub Hajizadeh, Karim EbrahimpourDocument5 pagesAtmospheric Pollution Research: Iman Parseh, Hakimeh Teiri, Yaghoub Hajizadeh, Karim EbrahimpourMaharghya BiswasNo ratings yet
- Determine Best Design Based on Annual ReturnDocument1 pageDetermine Best Design Based on Annual ReturnMaharghya BiswasNo ratings yet
- 07 9.4.2018Document7 pages07 9.4.2018Maharghya BiswasNo ratings yet
- Environmental Pollution: James D. Blande, Katariina Turunen, Jarmo K. HolopainenDocument7 pagesEnvironmental Pollution: James D. Blande, Katariina Turunen, Jarmo K. HolopainenMaharghya BiswasNo ratings yet
- Heritage Theme Resort: Thesis ReportDocument8 pagesHeritage Theme Resort: Thesis ReportNipun ShahiNo ratings yet
- Kone ErrorDocument2 pagesKone Errorrully hidayatullah50% (2)
- Plant SimulationDocument3 pagesPlant SimulationGrant Schorsch KalilNo ratings yet
- Cyril Acott - Occultism - An Alternative To Scientific HumanismDocument20 pagesCyril Acott - Occultism - An Alternative To Scientific Humanismparadigmshifter6360100% (2)
- Chapter 2 ClimateDocument21 pagesChapter 2 ClimateShahyan bilalNo ratings yet
- Sem-1 Drama: Do You Consider It As A Tragic Play? Submit Your Review. (Document20 pagesSem-1 Drama: Do You Consider It As A Tragic Play? Submit Your Review. (Debasis ChakrabortyNo ratings yet
- wk8 Activity PresentationDocument13 pageswk8 Activity Presentationapi-280934506No ratings yet
- Homeopathy BrochureDocument2 pagesHomeopathy Brochuresrwelling67% (3)
- CERN Initial Letter For Yr 12Document2 pagesCERN Initial Letter For Yr 12AlexFryNo ratings yet
- Excel Spreadsheet SoftwareDocument22 pagesExcel Spreadsheet SoftwareJared Cuento TransfiguracionNo ratings yet
- Listening Cd1Document7 pagesListening Cd1Iulian Teodor0% (1)
- Møire 4.01 Docs (1993)Document15 pagesMøire 4.01 Docs (1993)VintageReadMeNo ratings yet
- Delete Entries On TRBAT and TRJOB Tables ..Document3 pagesDelete Entries On TRBAT and TRJOB Tables ..ssssssssssNo ratings yet
- Notification JNTU Anantapur Assistant Professor Posts PDFDocument7 pagesNotification JNTU Anantapur Assistant Professor Posts PDFNagabhushanaNo ratings yet
- Binary Classification MetricsDocument6 pagesBinary Classification MetricssharathdhamodaranNo ratings yet
- Composing SentencesDocument2 pagesComposing Sentencesapi-250296212No ratings yet
- Snorks Udl Lesson Plan-1Document4 pagesSnorks Udl Lesson Plan-1api-253110466No ratings yet
- Module 5 HMWRK Lesson 14Document2 pagesModule 5 HMWRK Lesson 14ReekhaNo ratings yet
- Wireless Controlled Smart Digital Energy Meter and Theft Control Using GSM With GUIDocument6 pagesWireless Controlled Smart Digital Energy Meter and Theft Control Using GSM With GUIMuhammad FarhanNo ratings yet
- Video WorksheetDocument9 pagesVideo Worksheetapi-316047658100% (1)
- Labsheet 1 (Int)Document3 pagesLabsheet 1 (Int)mail meNo ratings yet
- The Historic 7Th March Speech of Bangabandhu Sheikh Mujibur RahmanDocument31 pagesThe Historic 7Th March Speech of Bangabandhu Sheikh Mujibur RahmanSanzid Ahmed ShaqqibNo ratings yet
- Dubai Workshop RegistrationDocument2 pagesDubai Workshop RegistrationmfkmughalNo ratings yet
- Process States and Memory Management LabDocument8 pagesProcess States and Memory Management LabJámesNo ratings yet
- Solution Manual For Mathematics For EconomicsDocument42 pagesSolution Manual For Mathematics For EconomicsMarcia Smith0% (1)
- Dr. Muhammad Yousuf Sharjeel CV January 2018Document8 pagesDr. Muhammad Yousuf Sharjeel CV January 2018Anonymous ipgHCggSNo ratings yet
- Nikola Tesla: Mysterious Facts (Essay)Document2 pagesNikola Tesla: Mysterious Facts (Essay)DenisKisurkinNo ratings yet
- Unit 03 Techniques of Planning, Controlling and Automating Software ProcessDocument36 pagesUnit 03 Techniques of Planning, Controlling and Automating Software ProcessSajjan PaudelNo ratings yet
- FreePBX Installation GuideDocument6 pagesFreePBX Installation Guidetinhs2cop0% (1)
- IRL - Information Request List For Performance DiagnosticsDocument3 pagesIRL - Information Request List For Performance Diagnosticsd280299No ratings yet