Professional Documents
Culture Documents
10 Senyawa Kompleks Aplikom
Uploaded by
Fatimatuz zahroh0 ratings0% found this document useful (0 votes)
20 views4 pages10 senyawa kompleks dalam mata kuliah aplikom
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document10 senyawa kompleks dalam mata kuliah aplikom
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
20 views4 pages10 Senyawa Kompleks Aplikom
Uploaded by
Fatimatuz zahroh10 senyawa kompleks dalam mata kuliah aplikom
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 4
2.
3 Periodic Properties of Atoms
A valuable aspect of the arrangment of atoms on the basis of similar electronic configurations
within the periodic table is that an atom’s position provides information about its properties. Some
of these properties, and how they vary across periods and groups, are now discussed.
2.3.1 Ionization Energy
The ionization energy, also known as the ionization potential, is the energy required to
remove an electron from a gaseous atom or ion:
An+ (g) A(n+1)+ (g) + e- ionization energy (IE) = ∆U
where n = 0 (first ionization energy), n = 1 (second ionization energy), and so on.
As would be expected from the effects of shielding, the ionization energy varies with
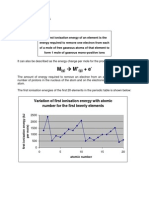
different nuclei and different numbers of electrons. Trends for the first ionization energies of the
early elements in the periodic table are shown in Figure 2.13. The general trend across a period
is an increase in ionization energy as the nuclear charge increases. However, the experimental
values show a break in the trend in the second period at boron and again at oxygen. Because boron
is the first atom to have an electron in a higher energy 2p orbital that is shielded somewhat by the
2s electrons, boron’s 2p electron is more easily lost than the 2s electrons of beryllium; boron has
the lower ionization energy.
(Gambar Konfigurasi Energy Be)
A t the fourth 2p electron, at oxygen, a similar decrease in ionization energy occurs. Here, the
fourth electron shares an orbital with one of the three previous 2p electrons
(Gambar atomic number)
(gambar spin), and the repulsion between the paired electrons (πc) reduces the energy necessary
to remove an electron from oxygen; oxygen has a lower ionization energy than nitrogen, which
has the 2p configuration.
Similar patterns appear in the other periods, for example Na through Ar and K through Kr,
omitting the transition metals. The transition metals have less dramatic differences in ionization
energies, with the effects of shielding and increasing nuclear charge more nearly in balance.
Much larger decreases in ionization energy occur at the start of each new period, because
the change to the next major quantum number requires that the new s electron have a much higher
energy. The maxima at the noble gases decrease with increasing Z , because the outer electrons
are farther from the nucleus in the heavier elements. Overall, the trends are toward higher
ionization energy from left to right in the periodic table (the major change) and lower ionization
energy from top to bottom (a minor change). The differences described in the previous paragraph
are superimposed on these more general changes.
2.3.2 Electron Affinity
Electron affinity can be defined as the energy required to remove an electron from a negative ion:
A- (g) A (g) + e- electron affinity (EA) = ∆U
Because of the similarity of this reaction to the ionization for an atom, electron affinity is
sometimes described as the zeroth ionization energy . This reaction is endothermic (positive ∆U
) except for the noble gases and the alkaline earth elements. The pattern of electron affinities with
changing Z, shown in Figure 2.13, is similar to that of the ionization energies, but for one larger
Z value (one more electron for each species) and with much smaller absolute numbers. For either
of the reactions, removal of the first electron past a noble gas configuration is easy, so the noble
gases have the lowest electron affinities. The electron affinities are all much smaller than the
corresponding ionization energies, because electron removal from a negative ion (that features
more shielding of the nuclear charge) is easier than removal from a neutral atom.
Comparison of the ionization and electron affinity graphs in Figure 2.13 shows similar zigzag
patterns, but with the two graphs displaced by one element: for example, electron affinity shows a
peak at F and valley at Ne, and ionization energy a peak at Ne and valley at Na.
(Gambar number of electrons)
The patterns in these two quantities can more easily be seen by plotting energy against the number
of electrons in each reactant, as shown in Figure 2.14 for electron affinity and first and second
ionization energy.
The peaks and valleys match for all three graphs because the electron configurations match—for
example, there are peaks at 10 electrons and valleys at 11 electrons. At 10 electrons, all three
reactant species (F–, Ne, and Na+) have identical 1s2 2s2 2p6 configurations; these are by definition
isoelectronic species. The relatively high energy necessary to remove an electron from these
configurations is typical for configurations in which electron shells are complete. The next
electron, in an 11-electron configuration, is the first to occupy a higher energy 3s orbital and is
much more easily lost, providing a valley in each graph, corresponding to removal of an electron
from the 11-electron species Ne – , Na, and Mg + .
2.3.3 Covalent and Ionic Radii
The sizes of atoms and ions are also related to the ionization energies and electron affinities. As
the nuclear charge increases, the electrons are pulled in toward the c enter of the atom, and the size
of any particular orbital decreases. On the other hand, as the nuclear charge increases, more
electrons are added to the atom, and their mutual r epulsion keeps the outer orbitals large. The
interaction of these two effects, increasing nuclear charge and increasing number of electrons,
results in a gradual decrease in atomic size across each period. Table 2.8 gives nonpolar covalent
radii, based on bond distances in nonpolar m olecules. There are other measures of atomic size,
such as the van der Waals radius, in which collisions with other atoms are used to define the size.
It is difficult to obtain consistent data for any such measure, because the polarity, chemical
structure, and physical state of molecules change drastically from one compound to another. The
numbers shown here are sufficient for a general comparison of different elements.
There are similar challenges in determining the size of ions. Because the stable ions of the different
elements have different charges and different numbers of electrons, as well as different crystal
structures for their compounds, it is difficult to find a suitable set of numbers for comparison.
Earlier data were based on Pauling’s approach, in which the ratio of the radii of isoelectronic ions
was assumed to be equal to the ratio of their effective nuclear charges.
(Gambar tabel 2.8)
(Gambar tabel 2.9)
More recent calculations are based on a number of considerations, including electron density maps
from X-ray data that show larger cations and smaller anions than those previously found. Those in
Table 2.9 and Appendix B-1 were called “crystal radii” by Shannon 22 and are generally different
from the older values of “ionic radii” by +14 pm for cations and –14 pm for anions, as well as
being revised to accommodate more recent measurements. The radii in Table 2.9 and Appendix
B-1 can be used for rough estimation of the packing of ions in crystals and other calculations, as
long as the “fuzzy” nature of atoms and ions is kept in mind.
Factors that influence ionic size include the coordination number of the ion, the covalent character
of the bonding, distortions of regular crystal geometries, and delocalization of electrons (metallic
or semiconducting character , described in Chapter 7) . The radius of the anion is also influenced
by the size and charge of the cation. Conversely, the anion exerts a smaller influence on the radius
of the cation. The table in Appendix B-1 shows the effect of coordination number.
The values in Table 2.10 show that anions are generally larger than cations with similar numbers
of electrons. The radius decreases as nuclear charge increases for ions with the same electronic
structure, with the charge on cations having a strong effect, for example in the series Na+, Mg2+,
Al3+ . Within a group, the ionic radius increases as Z increases because of the larger number of
electrons in the ions and, for the same element, the radius decreases with increasing charge on the
cation. Examples of these trends are shown in Tables 2.10, 2.11, and 2.12.
(Gambar tabel 2.10)
(Gambar tabel 2.11)
(Gambar tabel 2.12)
Translate Bahasa Indonesia
2.3 Sifat Periodik Atom
Aspek penting dari pengaturan atom berdasarkan konfigurasi elektron serupa dalam tabel periodik
yaitu bahwa posisi atom memberikan informasi tentang sifat-sifatnya. Beberapa sifatnyaini, dan
bagaimana mereka bervariasi di seluruh periode dan kelompok, sekarang akan dibahas.
2.3.1 Energi Ionisasi
Energi ionisasi (potensi ionisasi) adalah energi yang dibutuhkan untuk mengeluarkan elektron dari
atom atau ion gas:
An+ (g) A(n+1)+ (g) + e- energi ionisasi (IE) = ∆U
di mana n = 0 (energi ionisasi pertama), n = 1 (energi ionisasi kedua), dan seterusnya.
Seperti yang diharapkan dari efek shielding, energi ionisasi bervariasi dengan inti dan jumlah
elektron yang berbeda. Untuk energi ionisasi pertama dari unsur-unsur awal dalam tabel periodik
ditunjukkan pada Gambar 2.13.
(Gambar 2.13)
Kecenderungan umum di bagian periode adalah peningkatan energi ionisasi. Namun, nilai-nilai
eksperimen menunjukkan pemutusan pada periode kedua pada boron dan oksigen. Hal tersebut
tejadi karena boron adalah atom pertama yang memiliki elektron dalam energi tertinggi pada
orbital 2p yang terlindungi oleh elektron pada orbital 2s, elektron 2p pada Boron lebih mudah
hilang daripada elektron 2s pada Berilium; Boron memiliki energi ionisasi yang lebih rendah.
(Gambar Konfigurasi Energi Be)
Pada elektron 2p keempat, pada oksigen, terjadi penurunan serupa dalam energi ionisasi. Di sini,
elektron keempat berbagi orbital dengan salah satu dari tiga elektron 2p sebelumnya (gambar spin)
dan tolakan antara elektron berpasangan (πc) mengurangi energi yang diperlukan untuk
mengeluarkan elektron dari oksigen sehingga oksigen memiliki energi ionisasi yang lebih rendah
daripada nitrogen (yang memiliki konfigurasi 2p)
Pola serupa juga muncul pada periode lain, misalnya Na melalui Ar dan K melalui Kr, dalam
menghilangkan logam transisi. Logam transisi memiliki perbedaan yang kurang menggemparkan
dalam energi ionisasi, dengan efek shielding dan peningkatan muatan yang mengandung atom
yang hampir seimbang.
Penurunan energi ionisasi yang jauh lebih besar terjadi pada awal setiap periode baru, karena
perubahan ke nomor kuantum utama berikutnya mensyaratkan bahwa elektron baru memiliki
energi yang jauh lebih tinggi. Maksimum di gas mulia menurun dengan meningkatnya Z, karena
elektron terluar (yang jauh dari inti) dalam unsur-unsur yang lebih berat. Secara keseluruhan, tren
menuju energi ionisasi yang lebih tinggi dari kiri ke kanan dalam tabel periodik (perubahan besar)
dan energi ionisasi yang lebih rendah dari atas ke bawah (perubahan kecil). Perbedaan yang
dijelaskan dalam paragraf sebelumnya ditumpangkan pada perubahan yang lebih umum ini.
You might also like
- Ionization EnergyDocument69 pagesIonization EnergyVisalakshi Venkat100% (2)
- Ionization Energy Group 5Document7 pagesIonization Energy Group 5St. AnisaNo ratings yet
- Ion EnergiesDocument4 pagesIon EnergiesNamratha NairNo ratings yet
- Periodicity Trends Across Periods 2 and 3Document7 pagesPeriodicity Trends Across Periods 2 and 3Anshu MovvaNo ratings yet
- Ionization Energy and Electron AffinityDocument9 pagesIonization Energy and Electron AffinityKhan AaghaNo ratings yet
- Classification of ElementsDocument74 pagesClassification of ElementsJimit Patel BankNo ratings yet
- Muna Adam - Analyzing Ionization Energy DataDocument11 pagesMuna Adam - Analyzing Ionization Energy DataMuna AdamNo ratings yet
- Electron Configurations and PropertiesDocument28 pagesElectron Configurations and PropertiesAinthu IbrahymNo ratings yet
- CRYSTAL BINDING AND ELASTIC CONSTANTSDocument10 pagesCRYSTAL BINDING AND ELASTIC CONSTANTSSyifa'ul HasanahNo ratings yet
- HL Electron Configuration and The Periodic TableDocument12 pagesHL Electron Configuration and The Periodic TableMarilee HuntNo ratings yet
- Ionazation Energy GraphDocument15 pagesIonazation Energy GraphMaricel Cotanda EginaNo ratings yet
- Patterns of First Ionisation Energies in The Periodic TableDocument7 pagesPatterns of First Ionisation Energies in The Periodic TableRugen RajNo ratings yet
- Inorganic Chemistry NotesDocument105 pagesInorganic Chemistry NotesOdongo TonnyNo ratings yet
- What is a Mass SpectrometerDocument11 pagesWhat is a Mass SpectrometerFiliz Kocayazgan ShanablehNo ratings yet
- Ionization EnergyDocument2 pagesIonization EnergynapilgabethNo ratings yet
- Chapter 03 PeriodicityDocument116 pagesChapter 03 PeriodicityJishen ZhuNo ratings yet
- A-LEVEL INORGANIC CHEMISTRY NOTESDocument55 pagesA-LEVEL INORGANIC CHEMISTRY NOTESaudrey abaasaNo ratings yet
- Atomic Structure and Bonding v3cDocument34 pagesAtomic Structure and Bonding v3cBirdii97No ratings yet
- Ionisation Energy ClassDocument13 pagesIonisation Energy ClassKordell McShineNo ratings yet
- 4th ClassDocument11 pages4th Classvaibhav baluNo ratings yet
- Electron Configuration and Periodic PropertiesDocument48 pagesElectron Configuration and Periodic Propertiesahmad batataNo ratings yet
- 2013 Chemistry AnswerDocument22 pages2013 Chemistry Answerlevapo8821No ratings yet
- Review of Atomic Structure Atomic Bonding in Solids Primary Interatomic BondingDocument54 pagesReview of Atomic Structure Atomic Bonding in Solids Primary Interatomic BondingWilliams AkandiNo ratings yet
- IONISATION ENERGY PERIODIC TRENDDocument12 pagesIONISATION ENERGY PERIODIC TRENDhahajing1980No ratings yet
- A039level Chemistry Inorganic NotesDocument108 pagesA039level Chemistry Inorganic NotesNasser SsennogaNo ratings yet
- ChemistryDocument23 pagesChemistryYehualashet BelaynehNo ratings yet
- LoraineDocument29 pagesLoraineR I R INo ratings yet
- Semiconductor FundamentalsDocument18 pagesSemiconductor FundamentalsromfernNo ratings yet
- Chemical Bonding Narayana PDFDocument35 pagesChemical Bonding Narayana PDFggk201390% (10)
- Why atoms bond - electron configurations determine stabilityDocument21 pagesWhy atoms bond - electron configurations determine stabilityJawaid IqbalNo ratings yet
- As Level Chemistry NotesDocument113 pagesAs Level Chemistry NotesHARIS GAMINGNo ratings yet
- PhysRevA 46Document6 pagesPhysRevA 46amsterdam1963No ratings yet
- Ionization Energy 4Document4 pagesIonization Energy 4Neha esaralNo ratings yet
- Bonding in Metals and Semiconductors Explained by Band TheoryDocument20 pagesBonding in Metals and Semiconductors Explained by Band TheoryAhmat FananyNo ratings yet
- Chemistry Form 6 Sem 2 03Document45 pagesChemistry Form 6 Sem 2 03Ng Swee Loong StevenNo ratings yet
- A - Level Inorganic Chemistry 2-1Document171 pagesA - Level Inorganic Chemistry 2-1kitderoger_391648570No ratings yet
- Chapter - 1Document19 pagesChapter - 1api-314267144No ratings yet
- Chapter 2 (A) Enargy Band and Charge CarriersDocument4 pagesChapter 2 (A) Enargy Band and Charge CarriersvinodNo ratings yet
- Energy Bands and Charge Carriers in SemiconductorsDocument12 pagesEnergy Bands and Charge Carriers in SemiconductorsAshish Mg100% (1)
- Chapter 1, REVIEW OF QUONTAM THEORYDocument13 pagesChapter 1, REVIEW OF QUONTAM THEORYPAUL NDIRITUNo ratings yet
- Topic 1.1 Atomic Structure: 1.1a A Model of The AtomDocument5 pagesTopic 1.1 Atomic Structure: 1.1a A Model of The Atomking atikNo ratings yet
- GRADE 9 ReviewerDocument7 pagesGRADE 9 ReviewerMa Belle Jasmine DelfinNo ratings yet
- Bonga University: Engineering Material (Meng2091)Document30 pagesBonga University: Engineering Material (Meng2091)Mul'isaa JireenyaaNo ratings yet
- Chemistry 1311 Problem Set 1Document5 pagesChemistry 1311 Problem Set 1qabusalemNo ratings yet
- Module 5 Chemical BondsDocument23 pagesModule 5 Chemical BondsAjay WilliamsNo ratings yet
- Atomic Electron Configurations and PeriodicityDocument40 pagesAtomic Electron Configurations and PeriodicityYiTing TanNo ratings yet
- Atomic Radius and Effective Nuclear ChargeDocument8 pagesAtomic Radius and Effective Nuclear ChargeKisses EldswidthNo ratings yet
- Electron Configurations: Structure 1.3Document32 pagesElectron Configurations: Structure 1.3omarremch69No ratings yet
- As Chemistry 宝典 2023新版 Daisy-黑白5本 1Document80 pagesAs Chemistry 宝典 2023新版 Daisy-黑白5本 1bbcdgg1234567No ratings yet
- Periodic TrendsDocument9 pagesPeriodic TrendsSobia KashifNo ratings yet
- Unit - I P-N Junction Diode 1Document23 pagesUnit - I P-N Junction Diode 1Sandeep Babu VannempalliNo ratings yet
- AtomsDocument5 pagesAtomsjijigox479No ratings yet
- Chapter Eight BrownDocument13 pagesChapter Eight BrownJaka ShankalyanaNo ratings yet
- Periodic PropertiesDocument22 pagesPeriodic Propertiessecondary twoNo ratings yet
- Chem 111 Week Three Notes BDocument7 pagesChem 111 Week Three Notes BnyachiosirNo ratings yet
- Chapter 7 Jan13Document98 pagesChapter 7 Jan13kumuthaNo ratings yet
- Unit Objectives: Periodic TrendsDocument9 pagesUnit Objectives: Periodic Trendsmartin mulengaNo ratings yet
- Principles of Solar Cells, LEDs and Related Devices: The Role of the PN JunctionFrom EverandPrinciples of Solar Cells, LEDs and Related Devices: The Role of the PN JunctionNo ratings yet
- Electron Beam-Specimen Interactions and Simulation Methods in MicroscopyFrom EverandElectron Beam-Specimen Interactions and Simulation Methods in MicroscopyNo ratings yet
- As 128694 VHX-7000 C 601376 WW GB 2092 5Document40 pagesAs 128694 VHX-7000 C 601376 WW GB 2092 5Khairul AzharNo ratings yet
- Isotopes and Atomic StructureDocument9 pagesIsotopes and Atomic StructureClinton ChikengezhaNo ratings yet
- CH 1-Ch 7Document13 pagesCH 1-Ch 7Eligius MartinezNo ratings yet
- Che-01 IgnouDocument8 pagesChe-01 IgnouRamBabuMeenaNo ratings yet
- 2nd Q Grade 9 Science Budget of Work Sy 2019 2020Document1 page2nd Q Grade 9 Science Budget of Work Sy 2019 2020ArielNo ratings yet
- Chapter 2 Plane Surfaces and PrismsDocument22 pagesChapter 2 Plane Surfaces and PrismsEric Doctore KrageNo ratings yet
- YuzhaoMaDissertation PDFDocument161 pagesYuzhaoMaDissertation PDFTygerYashanshuNo ratings yet
- Study of Compound MicroscopeDocument4 pagesStudy of Compound MicroscopeArman KhanxNo ratings yet
- Guidelines - For - Clinical - Photography - 2015 Rev PDFDocument4 pagesGuidelines - For - Clinical - Photography - 2015 Rev PDFGualterio PoloNo ratings yet
- Damien Lovegrove - The Fujifilm X System Guide For Portrait Photographers-Damien Lovegrove (2016)Document92 pagesDamien Lovegrove - The Fujifilm X System Guide For Portrait Photographers-Damien Lovegrove (2016)NorAmalinaNo ratings yet
- Interference & Diffraction (Using A Laser)Document16 pagesInterference & Diffraction (Using A Laser)DevNo ratings yet
- Science7 Q2 M1A v4 No Key AnswerDocument11 pagesScience7 Q2 M1A v4 No Key AnswerCastolo Bayucot Jvjc100% (1)
- Convex Lens ExperimentDocument2 pagesConvex Lens Experimentayush kumarNo ratings yet
- Broadband Metamaterials in ElectromagneticsDocument399 pagesBroadband Metamaterials in ElectromagneticsFarhad AzadiNo ratings yet
- Jahn Teller Theorm 5th ChemDocument10 pagesJahn Teller Theorm 5th ChemMuhammad ArhamNo ratings yet
- Wave-Particle Duality of Electrons ExplainedDocument3 pagesWave-Particle Duality of Electrons ExplainedNyak Perera50% (2)
- QUARTER 1 LESSON 2 Atom Ions and MoleculesDocument60 pagesQUARTER 1 LESSON 2 Atom Ions and MoleculesMichelle De VillaNo ratings yet
- Chapter 3 Chemical Bonding and StructureDocument11 pagesChapter 3 Chemical Bonding and StructureTilak K CNo ratings yet
- STPM 2021 Sem 1 Mock AnsDocument3 pagesSTPM 2021 Sem 1 Mock Ansm-7319562No ratings yet
- Olympus CH - Brochure - OKDocument8 pagesOlympus CH - Brochure - OKYasuda ReiNo ratings yet
- Design and Numerical Analysis of A Circular SPR Based PCF Biosensor For Aqueous EnvironmentsDocument7 pagesDesign and Numerical Analysis of A Circular SPR Based PCF Biosensor For Aqueous Environmentsid.danlard5282No ratings yet
- Gamma RaysDocument34 pagesGamma RaysuzmaNo ratings yet
- 2004 Canon Bino BrochureDocument6 pages2004 Canon Bino BrochureCraig ThompsonNo ratings yet
- Camera Settings For Concert Photography BeginnersDocument22 pagesCamera Settings For Concert Photography Beginnersss_tayadeNo ratings yet
- Atomic Absorption Spectroscopy: Benny Alexander 1606831585 Jordan Andrean M 1606871032 Riski Winner L 1606836755Document27 pagesAtomic Absorption Spectroscopy: Benny Alexander 1606831585 Jordan Andrean M 1606871032 Riski Winner L 1606836755Farhan Rama DigitaNo ratings yet
- Principle:: A. DarkfieldDocument3 pagesPrinciple:: A. DarkfieldJonathanPayongayongNo ratings yet
- Full Download Test Bank For Introduction To Organic Chemistry 4th Edition Brown PDF Full ChapterDocument36 pagesFull Download Test Bank For Introduction To Organic Chemistry 4th Edition Brown PDF Full Chapterunseem.dulcino.sbd8f100% (18)
- PQCW ODFN 020 MWL01 Datasheet PDFDocument2 pagesPQCW ODFN 020 MWL01 Datasheet PDFEd Gar YundaNo ratings yet
- WS - 3 Class X Phy CH - 10 (Light - Refraction) - 1Document3 pagesWS - 3 Class X Phy CH - 10 (Light - Refraction) - 1Sankalp MishraNo ratings yet
- Optical InstrumentsDocument11 pagesOptical InstrumentsPankaj MishraNo ratings yet