Professional Documents
Culture Documents
Improved Version of The Fischer-Zach Synthesis of Glycals: Vitamin B-12 Catalyzed Reductive Elimination of Glycosyl Bromides
Uploaded by
scadvijayOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Improved Version of The Fischer-Zach Synthesis of Glycals: Vitamin B-12 Catalyzed Reductive Elimination of Glycosyl Bromides
Uploaded by
scadvijayCopyright:
Available Formats
1424 J. Org. Chem.
1999, 64, 1424-1425
Improved Version of the Fischer-Zach Scheme 1. Fischer-Zach Synthesis of Triacetyl
Synthesis of Glycals: Vitamin B-12 Glucal
Catalyzed Reductive Elimination of
Glycosyl Bromides
Christopher L. Forbes and Richard W. Franck *
Department of Chemistry, Hunter College,
New York, New York 10021-5024
Received September 28, 1998
Glycals are unsaturated sugars with a double bond
located between C1 and C2. These compounds have a
long history of use as building blocks in carbohydrate Scheme 2. B-12 Catalytic Cycle (Adapted from
chemistry, and if anything, their importance has in- Scheffold) for the Synthesis of Triacetyl Glucal
creased, particularly because of their effectiveness as
glycosyl donors.1,2 The first synthesis of glycals was
reported by Fischer and Zach, in which 1-bromo-tet-
racetylglucose was reduced with Zn dust in acetic acid.3
(Scheme 1).
This historic experiment served as the basis for the
modern method described in Methods in Carbohydrate
Chemistry and recently updated by Koreeda.4 A minor
but annoying technical problem in all versions of the
Fischer-Zach method is that extensive washing with
sodium bicarbonate is required during workup to neu-
tralize the acetic acid from the reaction medium. The one
flaw in the method is that it fails in the furanoid glycal
series where the acidic conditions lead to furan deriva-
tives. However, Ireland showed that a Na-naphthalene
modified version of the Fischer-Zach method of forming
anion radical reduction served to make furanoid glycals
glycals from glycosyl bromides, which is done in neutral
readily available.5 Over the years, other reducing systems
media and therefore avoids the multiple bicarbonate
have been used to prepare glycals from 1-bromopyranose
washes for removal of acetic acid, the reaction medium
materials.6 By and large, these new methods offer the
of the original procedure The modification is one de-
advantage of avoiding the acidic conditions of the Fis-
scribed by Scheffold in which vitamin B-12 is used as a
cher-Zach procedure. However, none have as yet been
catalyst for reductive eliminations.7 The best precedent
widely adopted in place of the original Zn dust method,
for our purposes was the use of B-12 with Zn dust to
probably because of a perception that the advantages to
cleave chloroethyl-O protecting groups.7c The vitamin is
be gained are outweighed by problems of special reagent
a source of cobalt(III), which is reduced to Co(I). The
preparation or manipulation. We wish to describe a
Co(I) is assumed to insert into the C-halogen bond,
(1) Danishefsky, S. J.; Bilodeau, M. T. Angew. Chem., Int. Ed. Engl.
which is fragmented by further reduction. In fact, a
1996, 35, 1380-1419. A recent review of glycal chemistry, with mixture of zinc dust and ammonium chloride can be used
particular emphasis on applications of glycosyl transfer in which to carry out the Co(III)-Co(I) transformation. Because
glycals are the donors, including extensive contributions of the
Danishefsky group.
the insertion-elimination step reoxidizes the B-12 back
(2) Marzabadi, C. H.; Dios, A.; Geer, A.; Franck, R. W. J. Org. Chem. to Co(III), only a catalytic amount of vitamin B-12 is
1998, 63, 6673-6679. An article that includes a survey of cycloaddition necessary for the reaction. (Scheme 2).
reactions of glycals.
(3) Fischer, E.; Zach, K. Sitzungsber. Kl. Preuss. Akad. Wiss. 1913, Thus, application of the Scheffold protocol, which uses
27, 311-317. methanol as solvent and ammonium chloride as a buffer
(4) . (a) Roth, W.; Pigman, W. Methods in Carbohydrate Chemistry along with Zn dust as a reductant, serves admirably in
Whistler, R. L., Wolfrom, M. L., Eds.; Academic Press: New York, 1963;
Vol. 2, p 405-408. (b) Shafizadeh, F. Methods in Carbohydrate the Fischer-Zach synthesis. The necessary control was
Chemistry; Whistler, R. L., Wolfrom, M. L., Eds., Academic Press: New run to show that without the vitamin B-12 the Zn dust/
York, 1963; Vol. 2, p 409-410. (c) Shull, B. K.; Wu, Z.; Koreeda, M. J. methanol combination gave glycal in poor yield. The
Carbohydr. Chem. 1996, 15, 955-964.
(5) Ireland, R. E.; Thaisrivongs, S.; Vanier, N.; Wilcox, C. S. J. Org. modification fails with furanoid glycals (as does the
Chem. 1980, 45, 48-61. original method). Table 1 lists the 1-bromosugar starting
(6) (a) DePouilly, P.; Chenede, A.; Mallet, J.-M.; Sinay, P. Bull. Soc. materials and yields of glycals formed. Because there is
Chim. Fr. 1993, 130, 256-265; SmI2. (b) Cavallaro, C. L.; Schwartz,
J. J. Org. Chem. 1995, 60, 7056; (Cp2TiCl)2; this article includes a
survey of reductive elimination methods. (c) Spencer, R. P.; Schwartz, (7) (a) Scheffold, R.; Rytz, G.; Walder, L. Modern Synthetic Methods;
J. Tetrahedron Lett. 1996, 37, 4357-4360; (Cp2TiCl)2. (d) Furstner, Scheffold, R., Salle, O., Eds.; Verlag: Frankfurt, 1983; Vol. 3, 355-
A.; Weidmann, H. J. Org. Chem. 1989, 54, 2307-2311; K-graphite. 440. (b) Scheffold, R.; Abrecht, S.; Orlinski, R.; Ruf, H.-R.; Stamoula,
Prof. Furstner, Max Planck Institut, Mulheim-Ruhr, <fuerstner@ P.; Tinembart, O.; Walder, L.; Weymuth, C. Pure Appl. Chem. 1987,
mpi-muelheim.mpg.de> has compiled an exhaustive listing of glycal 59, 363-372. (c) Scheffold, R.; Amble, E. Angew. Chem., Int. Ed. Engl.
syntheses via reductive elimination (personal communication from the 1980, 19, 629-630. (d) Zhou, D.-L.; Walder, P.; Scheffold, R.; Walder,
author). L. Helv. Chim. Acta 1992, 75 995-1011.
10.1021/jo981952e CCC: $18.00 © 1999 American Chemical Society
Published on Web 01/26/1999
Notes J. Org. Chem., Vol. 64, No. 4, 1999 1425
Table 1. Reactants, Products, and Yields in the Vitamin B-12 Catalyzed Elimination
reactant glycosyl bromide glycal yielda,b
tetraacetyl-1-bromo-D-glucose triacetyl-D-glucal 94% (60-70%) [98%]
tetraacetyl-1-bromo-D-galactose triacetyl-D-galactal 93% (66%) [58%]
triacetyl-1-bromo-L-rhamnose diacetyl-L-rhamnal 45% [71%]
tetraacetyl-1-bromo-D-mannose triacetyl-D-glucal 95%
a The yields in parenthesis are reported in the Methods in Carbohydrate Chemistry procedure (ref 4b). b The yields in brackets are
reported in the Koreeda procedure (ref 4c).
no difference in yield between the glucose and mannose crude product was dissolved in water (30 mL) and extracted with
examples, we conclude that stereoelectronic effects in this chloroform (1 × 60 mL and 3 × 30 mL). All of the organic
series are negligble. extracts were combined, dried over magnesium sulfate, concen-
trated via rotary evaporator, and dried under vacuum, at which
In conclusion, we believe that the Scheffold B-12 time a white solid appeared. The NMR of the product was
modification for Zn dust reductions can be profitably used identical to that of a commercial sample of pure tri-O-acetyl
in almost any case where Zn dust is used in reductive glucal. The product was obtained in 94% yield (0.708 g, 2.60
eliminations. mmol). This procedure was repeated to make tri-O-acetyl
galactal in 93% yield; the NMR of the galactal also was identical
Experimental Section to that of a commercial sample.
Preparation of 6-Deoxy-3,4 di-O-Acetoxy-L-Glucal (L-
The zinc used required successive washings with 1 N HCl, Rhamnal). Vitamin B-12 (82.4 mg, 0.06 mmol) was dissolved
water, and methanol for activation. It was then dried in vacuo in 20 mL of dry methanol under inert conditions, followed by
for at least 1 h. the addition of zinc dust (7.89 g, 120.7 mmol) and ammonium
Tetra-O-acetyl-D-glucopyranosyl bromide was purchased from chloride (6.48 g, 121.1 mmol). The solution was allowed to stir
Sigma Chemicals and was used to prepare tri-O-acetyl glucal. for 30 min, during which time it took on a deep red-purple color.
The other 1-bromosugars were obtained by means of the classical 6-Deoxy-2,3,4-tri-O-acetyl-L-glucosyl bromide (2.0327 g, 6.0 mmol)
HBr procedure, which in our hands gave better yields than the was then dissolved separately in 25 mL of dry methanol and
alternate TMSBr method. The glycal products were identified added to the original reaction flask. The resulting mixture
by direct comparison with authentic samples. became a yellow-orange color. After 5 min, the contents of the
Preparation of Tri-O-Acetyl Glucal. A solution of vitamin reaction flask were washed through Celite (using methanol) and
B-12 (36.7 mg, 0.027 mmol) in 10 mL of anhydrous methanol concentrated under reduced pressure. The reddish solid residue
was purged with N2 for 30 min in a three-necked 50-mL round- was then dissolved in water (70 mL) and extracted three times
bottom flask fitted with a stir bar. Then, the zinc (2.06 g, 31.5 with chloroform (70 mL each). The organic layers were collected,
mmol) and ammonium chloride (1.68 g, 31.5 mmol) were added dried over magnesium sulfate, and concentrated under reduced
to the solution, which was stirred for 45 min. Afterward, tetra- pressure. The resulting yellow runny oil was chromatographed
O-acetyl-D-glucopyranosyl bromide (1.07 g, 2.60 mmol) was on silica gel first pretreated with triethylamine 1% v/v in
dissolved in 5 mL of anhydrous methanol and added to the petroleum ether/ EtOAc 8:1 as a column rinsing solvent and then
reaction flask. Immediately after addition of the bromide, the eluted with petroleum ether/EtOAc 8:1. The isolated product was
dark red solution changed to reddish-yellow in color and then
a pure yellow oil (560 mg, 45%), identical by NMR with a
back to the dark red in about 30 s. A TLC was taken after 5
commercial sample.
min, which showed that the reaction had gone to completion (Rf
) 0.41 in 5:1 petroleum ether/ethyl acetate). The solution was
then filtered through a pad of Celite to remove the zinc, and Acknowledgment. This research was supported by
the Celite was rinsed with methanol to ensure that all of the NIH grants GM 51216 and RR 03037 (RCMI) and PSC-
solution was retrieved. The methanol solution was then concen- CUNY funds.
trated via rotary evaporator and vacuum dried to give the crude
white and red solid crude product. To isolate pure product, the JO981952E
You might also like
- Chemistry Structure and Properties 2nd Edition Tro Test BankDocument24 pagesChemistry Structure and Properties 2nd Edition Tro Test Bankjenniferrichardsonjrwfpzsdim100% (29)
- Formulating elixirs and calculating alcohol contentDocument16 pagesFormulating elixirs and calculating alcohol contentMikaela LaoNo ratings yet
- Reaksi PA Dan ButanolDocument5 pagesReaksi PA Dan ButanolUnlucky 2019No ratings yet
- 04 04 Roberts Target Ans1Document6 pages04 04 Roberts Target Ans1Albert BohrNo ratings yet
- Cubylglycine OBCDocument4 pagesCubylglycine OBCsevandhoustonNo ratings yet
- Org. Lett. 2008, 10, 2075. A Facile Preparation of Imidazolinium ChloridesDocument3 pagesOrg. Lett. 2008, 10, 2075. A Facile Preparation of Imidazolinium ChloridesDeiby ZambranoNo ratings yet
- 2005 OL OlefinesDocument3 pages2005 OL OlefinesCarmen SimalNo ratings yet
- Harzialactone TA 2005 LuDocument3 pagesHarzialactone TA 2005 LuhloicNo ratings yet
- Jones 1993Document2 pagesJones 1993kongaradamuNo ratings yet
- 961 Efficient Method Going From OH To Cle3b0Document3 pages961 Efficient Method Going From OH To Cle3b0Wolmir NemitzNo ratings yet
- Green Chemistry Approach To The Synthesis of N-Substituted Piperidones PDFDocument3 pagesGreen Chemistry Approach To The Synthesis of N-Substituted Piperidones PDFjavasoloNo ratings yet
- jiang2001Document5 pagesjiang2001Chiến NguyễnNo ratings yet
- Radical-Mediated Bromination of Carbohydrate Derivatives: Searching For Alternative Reaction Conditions Without Carbon TetrachlorideDocument4 pagesRadical-Mediated Bromination of Carbohydrate Derivatives: Searching For Alternative Reaction Conditions Without Carbon TetrachlorideBalaji ChandrasekharNo ratings yet
- Two Methods For The Preparation of Sitagliptin PhoDocument5 pagesTwo Methods For The Preparation of Sitagliptin PhoNadir BashirNo ratings yet
- Basudeb Basu Et Al - A Simple Protocol For Direct Reductive Amination of Aldehydes and Ketones Using Potassium Formate and Catalytic Palladium AcetateDocument3 pagesBasudeb Basu Et Al - A Simple Protocol For Direct Reductive Amination of Aldehydes and Ketones Using Potassium Formate and Catalytic Palladium AcetateRoundSTICNo ratings yet
- Acid-Catalyzed Solvent-Free Synthesis of 2-Arylbenzimidazoles Under MW J Mol Cat 2007Document4 pagesAcid-Catalyzed Solvent-Free Synthesis of 2-Arylbenzimidazoles Under MW J Mol Cat 2007mallari_naikNo ratings yet
- Synthesis of The Algicide BacillamideDocument7 pagesSynthesis of The Algicide Bacillamiderajesh kothariNo ratings yet
- An Improved Asymmetric Synthetic Route To D-Biotin Via Hoffmann-Roche Lactone-Thiolactone ApproachDocument4 pagesAn Improved Asymmetric Synthetic Route To D-Biotin Via Hoffmann-Roche Lactone-Thiolactone ApproachPaulo HenriqueNo ratings yet
- Arkivoc 2018, I, AmidinasDocument44 pagesArkivoc 2018, I, AmidinasgokucharlyNo ratings yet
- 09 Chapter 3Document36 pages09 Chapter 3cpunxzatawneyNo ratings yet
- Nakanishi 2014Document7 pagesNakanishi 2014Abhishek PareekNo ratings yet
- Alkylation PdDocument4 pagesAlkylation PdAbdelmajid farisNo ratings yet
- BENZOFURANOSDocument3 pagesBENZOFURANOSllòpez_737911No ratings yet
- Iodide-Catalyzed Reductions: Development of A Synthesis of Phenylacetic AcidsDocument6 pagesIodide-Catalyzed Reductions: Development of A Synthesis of Phenylacetic AcidsMike Roller100% (1)
- Chemistry of Peptide SynthesisDocument8 pagesChemistry of Peptide SynthesisNARESHKUMAR M BIONo ratings yet
- Pivaloyl chloride/DMF reagent converts alcohols to chloridesDocument3 pagesPivaloyl chloride/DMF reagent converts alcohols to chloridesalchymystNo ratings yet
- 2 CarbomethoxytropinoneDocument10 pages2 CarbomethoxytropinoneAnnana Myss100% (1)
- Borohydride IodineDocument4 pagesBorohydride IodineBandita DattaNo ratings yet
- Silica Sulfuric Acid An Efficient and Reusable Catalyst For The One-Pot Synthesis of 3,4-Dihydropyrimidin-2 (1H) - OnesDocument3 pagesSilica Sulfuric Acid An Efficient and Reusable Catalyst For The One-Pot Synthesis of 3,4-Dihydropyrimidin-2 (1H) - OnesDuy Phuc LeNo ratings yet
- NorrisolidetDocument4 pagesNorrisolidetOscar Martin OrdoñezNo ratings yet
- Poon 2006Document5 pagesPoon 2006NikaNo ratings yet
- s00706 008 0875 720160625 5071 11ib3hs With Cover Page v2Document4 pagess00706 008 0875 720160625 5071 11ib3hs With Cover Page v2mohamedNo ratings yet
- The Synthesis of GemcitabineDocument49 pagesThe Synthesis of GemcitabineAcih AsihNo ratings yet
- Cesium Carbonate S 2004 834787Document2 pagesCesium Carbonate S 2004 834787transitory.reificationNo ratings yet
- Glyconanoparticles Activity 3Document3 pagesGlyconanoparticles Activity 3ЛуизАпазаТ.No ratings yet
- Communications: A Practical Synthesis of - OseltamivirDocument3 pagesCommunications: A Practical Synthesis of - OseltamivirTom ChanNo ratings yet
- Synthesis, Characterization, Cytotoxicity, and AntibacterialDocument8 pagesSynthesis, Characterization, Cytotoxicity, and AntibacterialmisganaNo ratings yet
- Diazotazione NaNO2 NaHSO3Document3 pagesDiazotazione NaNO2 NaHSO3leda_prandiNo ratings yet
- 11d78440-2efa-4cb5-97ea-f14b881006e9 (1) (1)Document10 pages11d78440-2efa-4cb5-97ea-f14b881006e9 (1) (1)Dr Mohammed Elalaoui BelghitiNo ratings yet
- Marza I Oli 2012Document6 pagesMarza I Oli 2012Wulan AgustinaNo ratings yet
- David J. Dale Et Al - The Chemical Development of The Commercial Route To Sildenafil: A Case HistoryDocument6 pagesDavid J. Dale Et Al - The Chemical Development of The Commercial Route To Sildenafil: A Case HistoryRoundSTICNo ratings yet
- DDQ ReagentDocument7 pagesDDQ ReagentUmendra Khokhar100% (1)
- 4 Phenylbut 3 en 2 One 5Document3 pages4 Phenylbut 3 en 2 One 5Hóa Học Thạc SỹNo ratings yet
- CyclodextrinDocument8 pagesCyclodextrinAhmed HashmiNo ratings yet
- Organic Chem Highlights (2005)Document126 pagesOrganic Chem Highlights (2005)Xiaofeng MengNo ratings yet
- 1 s2.0 S000862150000046X MainDocument9 pages1 s2.0 S000862150000046X MainIsa Guerrero TroyanoNo ratings yet
- Benzo 04Document7 pagesBenzo 04hgdn701No ratings yet
- Coumarin Synth - Mild and High-Yielding Synthesis of B-Keto Esters and B-KetoamidesDocument6 pagesCoumarin Synth - Mild and High-Yielding Synthesis of B-Keto Esters and B-KetoamidesyunusNo ratings yet
- 2-Amidinylindole-3-Carbaldehydes: Versatile Synthons For The Preparation of A-Carboline DerivativesDocument7 pages2-Amidinylindole-3-Carbaldehydes: Versatile Synthons For The Preparation of A-Carboline DerivativesWalid EbaiedNo ratings yet
- CR 100258 KDocument35 pagesCR 100258 KzoyudgNo ratings yet
- Syntheses of The Enantiomers of 1-Deoxynojirimycin and 1-Deoxyaltronojirimycin Via Chemo-And Diastereoselective Olefinic Oxidation of Unsaturated AminesDocument14 pagesSyntheses of The Enantiomers of 1-Deoxynojirimycin and 1-Deoxyaltronojirimycin Via Chemo-And Diastereoselective Olefinic Oxidation of Unsaturated AminesDiogo DiasNo ratings yet
- Importante-Anhidrido CHO Generan Multiheterociclo en CascadaDocument8 pagesImportante-Anhidrido CHO Generan Multiheterociclo en CascadaFernando RSNo ratings yet
- Imes e Idipp SinteseDocument12 pagesImes e Idipp SinteseRodolpho NestaNo ratings yet
- Alcohol Inversion: Beyond The MitsunobuDocument4 pagesAlcohol Inversion: Beyond The MitsunobudoubleffectNo ratings yet
- The Photodecomposition of BromacilDocument6 pagesThe Photodecomposition of BromacilSh1vaNo ratings yet
- OPRD - Optimization of Manufacturing Route To PF-610355Document11 pagesOPRD - Optimization of Manufacturing Route To PF-610355rrgodboleNo ratings yet
- Rapid Access To Novel 2-Alkylthiopyrimidine Derivatives and Attempt of Their Tacrine Analogs SynthesisDocument10 pagesRapid Access To Novel 2-Alkylthiopyrimidine Derivatives and Attempt of Their Tacrine Analogs SynthesisBi LouNo ratings yet
- Anthony R. Lingham Et Al - Studies Towards The Synthesis of Salvinorin ADocument9 pagesAnthony R. Lingham Et Al - Studies Towards The Synthesis of Salvinorin ABic0000No ratings yet
- C-Glycosyl Compounds in The Synthesis of The Phytotoxin DiplopyroneDocument20 pagesC-Glycosyl Compounds in The Synthesis of The Phytotoxin DiplopyroneHannah CurranNo ratings yet
- Sustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeFrom EverandSustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeNo ratings yet
- Aliphatic Compounds: Trihydric Alcohols, Their Oxidation Products and Derivatives, Penta- and Higher Polyhydric Alcohols, Their Oxidation Products and Derivatives; Saccharides, Tetrahydric Alcohols, Their Oxidation Products and DerivativesFrom EverandAliphatic Compounds: Trihydric Alcohols, Their Oxidation Products and Derivatives, Penta- and Higher Polyhydric Alcohols, Their Oxidation Products and Derivatives; Saccharides, Tetrahydric Alcohols, Their Oxidation Products and DerivativesNo ratings yet
- Transition Metal-Catalyzed Pyridine Synthesis: Transition Metal-Catalyzed Heterocycle Synthesis SeriesFrom EverandTransition Metal-Catalyzed Pyridine Synthesis: Transition Metal-Catalyzed Heterocycle Synthesis SeriesNo ratings yet
- Toluidine To BromotolueneDocument4 pagesToluidine To BromotoluenescadvijayNo ratings yet
- Ese Dec 2018 PDFDocument1 pageEse Dec 2018 PDFscadvijayNo ratings yet
- ChemkeyRep Dec2018 PDFDocument3 pagesChemkeyRep Dec2018 PDFscadvijayNo ratings yet
- 2.24 Pyrans and Fused Pyrans: (Iii) Synthesis and ApplicationsDocument1 page2.24 Pyrans and Fused Pyrans: (Iii) Synthesis and ApplicationsscadvijayNo ratings yet
- RearrangementsDocument115 pagesRearrangementsscadvijayNo ratings yet
- Retrosynthesis SolutionsDocument7 pagesRetrosynthesis SolutionsScott Hendricks100% (1)
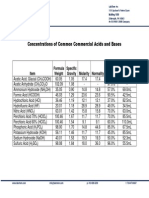
- Concentrations of Common Commercial Acids and BasesDocument1 pageConcentrations of Common Commercial Acids and BasesNazimah MaqboolNo ratings yet
- Concentrations of Common Commercial Acids and BasesDocument1 pageConcentrations of Common Commercial Acids and BasesNazimah MaqboolNo ratings yet
- 2 Nuclear Magnetic ResonanceDocument8 pages2 Nuclear Magnetic ResonanceSourav Kumar Jena100% (2)
- Analysis 1 PDFDocument17 pagesAnalysis 1 PDFscadvijayNo ratings yet
- Shift ReagentsDocument6 pagesShift ReagentsscadvijayNo ratings yet
- Bromination of AcetanilideDocument2 pagesBromination of AcetanilidescadvijayNo ratings yet
- Atomic OrbitalsDocument19 pagesAtomic OrbitalsOracle1511No ratings yet
- Liquid Ammonia As A Solvent 2Document3 pagesLiquid Ammonia As A Solvent 2scadvijayNo ratings yet
- Cla TH RatesDocument13 pagesCla TH RatesscadvijayNo ratings yet
- Molecules.: - Intermolecular Forces Are Interactions That Exist BetweenDocument14 pagesMolecules.: - Intermolecular Forces Are Interactions That Exist BetweenscadvijayNo ratings yet
- Elemental Sulfur and Sulfur-Rich Compounds II. Topics in Current Chemistry, 231Document3 pagesElemental Sulfur and Sulfur-Rich Compounds II. Topics in Current Chemistry, 231scadvijayNo ratings yet
- 11 Chemistry Notes ch12 Organic Chemistry PDFDocument3 pages11 Chemistry Notes ch12 Organic Chemistry PDFRangbaaz DA FIRENZENo ratings yet
- BPE Technical DataDocument5 pagesBPE Technical Datasmtamaskar2277No ratings yet
- Test 2 CHM572 June 2024Document3 pagesTest 2 CHM572 June 2024NUR AINA SYAHMINA MOHD AMRANNo ratings yet
- Scientific Paper Exp 5Document4 pagesScientific Paper Exp 5Brent TenorioNo ratings yet
- ISO 17025 NDT Lab Accreditation CertificateDocument3 pagesISO 17025 NDT Lab Accreditation Certificatekiki270977No ratings yet
- Power-to-Steel Study: Steel-Making Systems To Reduce CO Emissions by Utilizing Renewable Energy in The German Steel IndustryDocument10 pagesPower-to-Steel Study: Steel-Making Systems To Reduce CO Emissions by Utilizing Renewable Energy in The German Steel IndustryEvira Bella YustianiNo ratings yet
- Demulsifiers For Simulated Basrah Crude OilDocument107 pagesDemulsifiers For Simulated Basrah Crude OilTuan Yusoff100% (2)
- Organic Chemistry Compounds 9Document30 pagesOrganic Chemistry Compounds 9silvio1980No ratings yet
- SDS Hydrochloric Acid InhibitorDocument4 pagesSDS Hydrochloric Acid InhibitorJahidul IslamNo ratings yet
- 8 Msds MSDSDocument21 pages8 Msds MSDSoddyNo ratings yet
- Salt Analysis: Detecting Anions and CationsDocument9 pagesSalt Analysis: Detecting Anions and CationsAyush MukherjeeNo ratings yet
- 06chapters13 15Document19 pages06chapters13 15Achmad Arifudin HidayatullohNo ratings yet
- Rancidity in FoodDocument3 pagesRancidity in FoodNur Ain Nadiah AbahaNo ratings yet
- Review Organic AcidsDocument27 pagesReview Organic Acidscleon79100% (2)
- WPS SumDocument61 pagesWPS SumA. ΒρατσισταNo ratings yet
- Natural Gums and Its Pharmaceutical ApplicationDocument10 pagesNatural Gums and Its Pharmaceutical Applicationhosam alosNo ratings yet
- TautomerismDocument2 pagesTautomerismZIdanNo ratings yet
- Structures and Properties of CeramicsDocument38 pagesStructures and Properties of CeramicsArjayNo ratings yet
- Stability of Food Emulsions (2) : David Julian McclementsDocument37 pagesStability of Food Emulsions (2) : David Julian McclementsscribdGMMEGANo ratings yet
- Masterprotect 190: A Two Component Solvent Free High Build Flexible Epoxy Polyurethane Resin Coating SystemDocument3 pagesMasterprotect 190: A Two Component Solvent Free High Build Flexible Epoxy Polyurethane Resin Coating SystemEngTamer100% (1)
- 2 - ArrowPushingDocument14 pages2 - ArrowPushingYoung GothNo ratings yet
- Vocabulary Practice: Chapter 2 - Chemistry of LifeDocument5 pagesVocabulary Practice: Chapter 2 - Chemistry of LifeJordyNo ratings yet
- Engineering materials properties guideDocument5 pagesEngineering materials properties guideRam SwaroopNo ratings yet
- Effect of PH On Corrosion RateDocument8 pagesEffect of PH On Corrosion RateياسرشلالالحسنيNo ratings yet
- Mechanical SealsDocument16 pagesMechanical SealsGromin' AroundNo ratings yet
- Form 1 Science - Unit 3.3: The Concept of DensityDocument1 pageForm 1 Science - Unit 3.3: The Concept of DensitySuhaila SaniNo ratings yet
- EHS 1001 Compliance FormsDocument18 pagesEHS 1001 Compliance FormsAndre Santos SantosNo ratings yet
- Enzymatic Desizing of Cotton FabricsDocument7 pagesEnzymatic Desizing of Cotton Fabricseshaniqbal100% (1)