Professional Documents
Culture Documents
MFR AppNotes
Uploaded by
Vinh Do ThanhOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
MFR AppNotes
Uploaded by
Vinh Do ThanhCopyright:
Available Formats
SmarResearch
TechnologySource
HART®
Fieldbus
Profibus
Intrinsic Safety
Configuration Tools
Mass Flow Rate
Semiconductors
Training Determination for
Custom Design
Multivariable Transmitter
(Application Notes)
V 1.0
Visit the SmarResearch
technology center at:
www.smarresearch.com
©Smar
Research Corporation 1 MFR-0902
Mass Flow Rate - Table of Contents
Mass Flow Rate - Table of Contents
Topic Page Number
I. Symbols and Definitions 3
II. Necessary Specifications 4
III. Parameters 5
IV. Mass Flow Equation 5
V. Determination of Diameters/Diameter Ratio/Velocity of Approach 6
VI. Determination of Expansion Factor 6
VII. Determination of Isentropic Exponent 7
VIII. Determination of Fluid Density 8
IX. Determination of Viscosity 9
X. Determination of Coefficient of Discharge
AGA3 Orifice Plates 10
ISO Orifice Plates 11
ASME Orifice Plates 12
Nozzle, ISA 1932, ISO 12
Nozzle, Long Radius Wall Taps, ISO 12
Nozzle, Long Radius Wall Taps, ASME 12
Venturi Nozzle, ISO 13
Venturi, Rough Cast Inlet, ISO 13
Venturi, Rough Cast Inlet, ASME 13
Venturi, Machined Inlet, ISO 13
Venturi, Machined Inlet, ASME 13
Venturi, Welded Inlet, ISO 13
Small Bore Orifice Plate, Flange Taps, ASME 14
Appendix A: Sample of AIChE/DIPPR Database 15
Appendix B: Formulation of Isentropic Exponent of Steam Approximation 16
Appendix C: Formulation of Steam Viscosity Approximation 18
Appendix D: Custom Liquid Calculations 20
Custom Gas Calculations 21
Appendix E: Natural Gas Compressibility Equations 22
Appendix F: Iterative Process Used to Solve for Coefficient of Discharge 31
Appendix G: References 32
©Smar Research Corporation 2 MFR-0902
Mass Flow Rate - Symbols and Definitions
I. Symbols and Definitions
Symbol Definition
Cd Coefficient of discharge
Cp Specific heat at constant pressure
d Throat diameter at flowing temperature
davg Throat diameter at average temperature
dr Throat diameter at reference temperature
D Pipe diameter at flowing temperature
Davg Pipe diameter at average temperature
Dc Derivative of the correlation value of C d
Dr Pipe diameter at reference temperature
Ev Velocity of approach factor
Fc Correlation value of C d
Gi Ideal gas relative density
k Isentropic exponent
L1 Upstream tap position
L2 Downstream tap position
Mrair Molecular weight of air
Mw Molecular weight
Pc Critical pressure
Pf Flowing pressure
Qm Mass flow rate
R Universal gas constant
Re Reynolds number
Tavg Average temperature/Median value of specified temperature range
Tc Critical temperature
Tf Flowing temperature
Tr Reference temperature
v Specific volume
x Ratio of differential pressure to flowing pressure
Y Fluid expansion factor
Zf Compressibility at flowing conditions
α1 Linear coefficient of thermal expansion of primary element
α2 Linear coefficient of thermal expansion of pipe
β Ratio of throat diameter to pipe diameter
∆P Differential pressure
µf Absolute viscosity of flowing fluid
ρf Density of fluid at flowing temperature
©Smar Research Corporation 3 MFR-0902
Mass Flow Rate - Necessary Specifications
II. Necessary Specifications
Specify Fluid as:
a. Gas (from AIChE/DIPPR database)
b. Liquid (from AIChE/DIPPR database)
c. Steam
d. Natural Gas (3 options)
1. Gross Characterization Method
- Mole fraction of all components
2. Detail Characterization Method #1
- Real gas relative density
- Volumetric gross heating value
- Mole fraction of carbon dioxide
3. Detail Characterization Method #2
- Real gas relative density
- Mole fraction of carbon dioxide
- Mole fraction of nitrogen
e. Custom Fluid
- See Appendix D
Specify Primary Element as:
a. Orifice, Flange Taps (ISO, ASME, AGA3)
b. Orifice, Corner Taps (ISO, ASME)
c. Orifice, D & D/2 Taps (ISO, ASME)
d. Small Bore Orifice, Flange Taps, ASME
e. Nozzle, Long Radius Wall Taps (ISO, ASME)
f. Nozzle, ISA 1932, ISO
g. Venturi Nozzle, ISO
h. Venturi, Rough Cast Inlet (ISO, ASME)
i. Venturi, Machined Inlet (ISO,ASME)
j. Venturi, Welded Inlet, ISO
Specify Pipe/Throat:
- Diameters at reference temperature
- Materials
Specify Operating Range:
- Temperature (Tmin , Tmax)
- Pressure (P min , Pmax)
©Smar Research Corporation 4 MFR-0902
Mass Flow Rate - Parameters/Equation
III. Parameters
Gas/Liquid Temperature Range: Tf = 300 - 1500 F (-184.4 - 815.6 C)
Steam Temperature Range: Tf = Tsat - 1500 F (Tsat - 815.6 C)
Natural Gas Temperature Ranges:
Detail Method Tf = -200 - 400 F (-128.9 - 204.4 C)
Gross Methods Tf = 32 - 130 F (0- 54.4 C)
Absolute Pressure Range: Pf = 0.5 – 3626 psia
Pf = 3.447 - 25000.4 kPa
Pf = 0.034 – 250 bar
Differential Pressure Range: ∆P = 0 – 830 inH2O (0 - 206 kPa)
Pressure Ratio (x = ∆P/P f) x < 0.25
Natural Gas Parameters
Ideal gas relative density Gi = 0.554 - 0.87
Vol gross heating value: HV = 477 - 1150 Btu/ft3 (18.7 - 45.1 MJ/m3)
Mole fraction CO2 xCO2 = 0 - 30 %
Mole fraction N2 xN2 = 0 - 50 %
Each primary element provides different parameters for the following:
Pipe diameter (D)
Throat diameter (d)
Diameter ratio (β)
Reynolds number (Re)
IV. Mass Flow Equation
π 2
Qm Ev Cd d Y 2ρ f ∆ P
4
Cd = coefficient of discharge
d = throat diameter at flowing temperature
Ev = velocity of approach factor
Qm = mass flow rate
Y = fluid expansion factor
∆P = differential pressure
ρ f = density of fluid at flowing temperature
©Smar Research Corporation 5 MFR-0902
Mass Flow Rate - Determination of Diameter/Expansion Factor
V. Determination of Diameters/Diameter Ratio/Velocity Approach
d dr 1 α 1 Tf Tr D Dr 1 α 2 Tf Tr
d = throat diameter at flowing temperature
dr = throat diameter at reference temperature
D = pipe diameter at flowing temperature
Dr = pipe diameter at reference temperature
Tf = flowing temperature
Tr = reference temperature
α 1 = linear coefficient of thermal expansion of primary element
α 2 = linear coefficient of thermal expansion of pipe
d avg
β
Davg
d avg dr 1 α 1 Tavg Tr Davg Dr 1 α 2 Tavg Tr
davg = throat diameter at average temperature
Davg = pipe diameter at average temperature
Tavg = average temperature/median value of specified temperature range
1
Ev
4
1 β
Ev = velocity of approach factor
VI. Determination of Expansion Factor
For Orifice Plates:
4
0.41 0.35 β ∆P
Y 1
k Pf
k = isentropic exponent
Pf = flowing pressure
∆P = differential pressure
Y = fluid expansion factor
For Nozzles or Venturi Tubes:
1
2
2 k 1
k.( 1
k 4 k
x) 1 β 1 (1 x)
Y
k 1 2 x
4 k
1 β (1 x)
x = ∆P/ P f
©Smar Research Corporation 6 MFR-0902
Mass Flow Rate - Determination of Isentropic Exponent
VII. Determination of Isentropic Exponent
For Gas:
Cp
k
Cp R
2 2
C E
Tavg Tavg
Cp A B D
C E
sinh cosh
Tavg Tavg
Cp = specific heat at constant pressure
R = universal gas constant
A, B, C, D, and E are constants provided by AIChE/DIPPR database
For Liquid:
Y = 1 therefore determination of k is not necessary
For Steam:
k is estimated using the following linear approximation:
k 1.33544 6.24543 . 10 5
Tavg
Data used to formulate this approximation is contained within Appendix B.
For Natural Gas:
k is estimated according to AGA Report 3 Part 4:
k = 1.3
©Smar Research Corporation 7 MFR-0902
Mass Flow Rate - Determination of Fluid Density
VIII. Determination of Fluid Density
For Gas:
Pf M w
ρf
Zf R Tf
Mw = molecular weight
Zf = compressibility at flowing conditions
Zf is calculated using the Redlich-Kwong equations of state:
v a
Zf
v b R . Tf
1.5
(v b)
2.5
2
Tc Tc
a .42748 R b .08664 R
Pc Pc
3
R . Tf 2 a 2
b . R . Tf a .b
v v b v =0
Pf Pf . Tf Pf Pf . Tf
.5 .5
Pc = critical pressure
Tc = critical temperature
v = specific volume
For Liquid:
A
ρf
D
T
f
1 1
C
B
A, B, C, and D are constants provided by AIChE/DIPPR database
For Steam:
ρf is calculated from Tables S-1 through S-5 from the ASME International Steam
Tables. Density is equal to the inverse of specific volume (v).
For Natural Gas:
Pf Mrair Gi
ρf
Zf R Tf
Gi = ideal gas relative density
Mrair = molecular weight of air
Zf is calculated using the natural gas compressibility equations from AGA Report 8.
These equations are contained in Appendix D.
©Smar Research Corporation 8 MFR-0902
Mass Flow Rate - Determination of Viscosity
IX. Determination of Viscosity
For Gas:
B
A Tf
µf
C D
1
Tf Tf
2
µ f = absolute viscosity of flowing fluid
A, B, C, and D are constants provided by AIChE/DIPPR database.
For Liquid:
B E
A Cln T D T
T f f
f
µf e
A, B, C, D, and E are constants provided by AIChE/DIPPR database.
For Steam:
µ f is estimated using the following linear approximation:
6
µ 7.51661 0.02249 Tf 10
f
Data used to formulate this approximation is contained within Appendix C.
For Natural Gas:
µ f is estimated according to AGA Report 3 Part 4:
µ f = 0.0000069 lbm/ft*sec or 0.010268 cP
©Smar Research Corporation 9 MFR-0902
Mass Flow Rate - Determination of Coefficient of Discharge
X. Determination of Coefficient of Discharge
***For these primary elements, C d is a function of Reynolds number, and Reynolds num-
ber is a function of the unknown mass flow. Therefore, C d can not be directly calculated
and it is necessary to guess values for C d and Reynolds number and use an iterative
process to find the exact values. A detailed description of this iterative process is con-
tained in Appendix D.
AGA3 Orifice Plates:
Flange taps Corner or D&D/2 taps
0.05m < D < 1m 0.05m < D < 1m
d > 0.0125m d > 0.0125m
0.1 < β < 0.75 0.1 < β < 0.75 D
Re > 4000(β<0.5) Re > 4000(β<0.5)
Re > 170,000Dβ 2(β>0.5) Re > 16,000β 2(β>0.5) d
Assume an initial value of 4000 for the Reynolds number to get the following equations:
4 1.3
8.5 L 6L β L2 L2
.0712 . e .1145 . e
2 8 1 1 1.1
Cd0 .5961 .0291 β .229 β .0433 .0232 .014853 β
1 β
4 1 β 1 β
For D < .0711m add following term to C d0:
D
.003 ( 1 β ) 2.8
.0254
0.7
Cd1 .0244 β
4
Cd2 .145 β
4.8
Cd3 .1177 β
4.8 1.3
8.5 L 6L β .0113 L2 L2
.057 . e .0916 . e 7.232 . 10
1 1 3 1.9
Cd4 .0346 β
1 β
4 1 β 1 β
Corner Pressure Taps:
L1 = L2 = 0
D and D/2 Pressure Taps:
L1 = 1 and L2 = 0.47
Flange Pressure Taps:
L1 = L2 = .0254/Davg
Calculate for X:
4000 Dµ
X
Cd0 . Ev Yd . 2 ρ f . ∆ P
2
©Smar Research Corporation 10 MFR-0902
Mass Flow Rate - Determination Coefficient of Discharge
Use Cd0-4 and X to solve the following equations:
0.35 0.8 0.35 0.8
Fc Cd0 Cd1 X Cd2 Cd3 X X Cd4 X
0.35 0.8 0.35 0.8
Dc 0.7 Cd1 X 0.35 Cd2 1.15 Cd3 X X 0.8 Cd4 X
Cd(1) = Cd0 - δCd
Cd0 Fc
δCd
Dc
1
Cd0
Recalculate X with new value of C d(1) in place of C d. Use this value of X to recal-
culate F c , D c, and δCd. Continue repeating this process until δCd < 5x10-6 .
ISO Orifice Plates***:
Corner taps Flange or D&D/2 taps
0.05m < D < 1m 0.05m < D < 1m
d > 0.0125m d > 0.0125m
0.2 < β < 0.75 0.2 < β < 0.75
D
Re > 5,000(β<0.45) Re > 1,260,000β 2D(β<0.45)
Re > 10,000(β>0.45) Re > 1,260,000β 2D(β>0.45) d
If L1 < 0.4333 then:
6 .75 4
2.1 8 2.5 10 β 3
Cd 0.5959 0.0312 β 0.184 β 0.0029 β 0.09 L1 0.0337 L2 β
Re 1 β
4
If L1 > 0.4333 then:
6 .75 4
2.1 8 2.5 10 β 3
Cd 0.5959 0.0312 β 0.184 β 0.0029 β 0.039 L1 0.0337 L2 β
Re 1 β
4
Corner Pressure Taps:
L1 = L2 = 0
D and D/2 Pressure Taps:
L1 = 1 and L2 = 0.47
Flange Pressure Taps:
L1 = L2 = .0254/Davg
©Smar Research Corporation 11 MFR-0902
Mass Flow Rate - Determination of Coefficient of Discharge
ASME Orifice Plates***:
Flange taps Corner or D&D/2 taps
0.0508m < D < 1m 0.05m < D < 1m
d > 0.0125m d > 0.0125m D
0.2 < β < 0.7 0.2 < β < 0.7
d
If L1 < 0.4333 then:
6 .75 4
2.1 8 2.5 10 β 3
Cd 0.5959 0.0312 β 0.184 β 0.0029 β 0.09 L1 0.0337 L2 β
Re 1 β
4
If L1 > 0.4333 then:
6 .75 4
2.1 8 2.5 10 β 3
Cd 0.5959 0.0312 β 0.184 β 0.0029 β 0.039 L1 0.0337 L2 β
Re 1 β
4
Corner Pressure Taps:
L1 = L2 = 0
D and D/2 Pressure Taps:
L1 = 1 and L2 = 0.47
Flange Pressure Taps:
L1 = L2 = .0254/Davg
Nozzle, ISA 1932, ISO***:
0.05m < D < 0.5m
0.3 < β < 0.8 D
7x104 < Re < 107(β < 0.44)
2x104 < Re < 107(β > 0.44) d
6 1.15
4.1 2 4.15 10
Cd 0.9900 0.2262 β 0.00175 β 0.0033 β
Re
Nozzle, Long Radius Wall Taps, ISO***:
0.05m < D < 0.63m
0.2 < β < 0.8 D
104 < Re < 107
d
6 0.5
10
0.00653 . β
.5 .
Cd 0.9965
Re
Nozzle, Long Radius Wall Taps, ASME***:
0.1m < D < 0.75m
0.2 < β < 0.8
D
104 < Re < 6x106
d
6 0.5
.5 10
Cd 0.9975 0.00653 . β .
Re
©Smar Research Corporation 12 MFR-0902
Mass Flow Rate - Determination of Coefficient of Discharge
Venturi Nozzle, ISO:
0.065m < D < 0.5m d
d > 0.05m
0.316 < β < 0.775
D
1.5x105 < Re < 2x106
4.5
Cd 0.9858 0.196 β
Venturi, Rough Cast Inlet, ISO:
0.1m < D < 0.8m d
0.3 < β < 0.75
2x105 < Re < 2x106
Cd = 0.984 D
Venturi, Rough Cast Inlet, ASME:
0.1m < D < 1.2m d
0.3 < β < 0.75
2x105 < Re < 6x106
Cd = 0.984 D
Venturi, Machined Inlet, ISO:
0.05m < D < 0.25m
0.4 < β < 0.75 d
2x105 < Re < 1x106
Cd = 0.995
D
Venturi, Machined Inlet, ASME:
0.05m < D < 0.25m
0.3 < β < 0.75 d
2x105 < Re < 2x106
Cd = 0.995
D
Venturi, Welded Inlet, ISO:
0.2m < D < 1.2m d
0.4 < β < 0.7
2x105 < Re < 2x106
Cd = 0.985 D
©Smar Research Corporation 13 MFR-0902
Mass Flow Rate - Determination of Coefficient of Discharge
Small Bore Orifice Plate, Flange Taps, ASME***:
0.025 < D < 0.04m
d > 0.006m
D
0.15 < β < 0.7
Re > 1000 d
4
4 12 4 4 . 1 β
Cd 0.598 0.468 β 10 β 1 β 0.87 8.1 β
Re
©Smar Research Corporation 14 MFR-0902
Mass Flow Rate - Appendix A
Appendix A
Sample of AIChE/DIPPR Database
Gas Database
Fluid Mw Pc Tc Cp Viscosity
A B C D E A B C D
Acetic Acid
Acetone
Acetonitrile
Acetylene
Acrylonitrile
Liquid Database
Fluid Density Viscosity
A B C D A B C D E
Acetic Acid
Acetone
Acetonitrile
Acetylene
Acrylonitrile
©Smar Research Corporation 15 MFR-0902
Mass Flow Rate - Appendix B
Appendix B
Formulation of Isentropic Exponent of Steam Approximation
According to the ASME Steam Tables, isentropic exponent is a function of both
temperature and pressure. Its value can be found by plotting temperature and pres-
sure on Figure 7 of the ASME Steam Tables and estimating the corresponding isen-
tropic exponent. The complexity of the graph makes it impossible to create a direct
formula for the calculation of isentropic exponent. Therefore, assumptions must be
made in order to simplify the process. Various software was then analyzed to deter-
mine what assumptions could be made.
It can be assumed that the isentropic exponent does not vary with changing
pressure, therefore it is only a function of temperature. To determine an equation for
isentropic exponent as a function of temperature, various values of temperature were
inputted into the software. The corresponding values of isentropic exponent were then
graphed and a trend line was formulated. The equation of this trend line would provide
values of isentropic exponent for all possible values of temperature.
The following table is the values of isentropic exponent provided by the software
at various temperatures:
Temp k Temp k Temp k Temp k
79.5855 1.32873 450 1.30801 825 1.28301 1200 1.26007
100 1.32806 475 1.30631 850 1.28141 1225 1.25863
125 1.32713 500 1.30461 875 1.27982 1250 1.25721
150 1.32608 525 1.30291 900 1.27824 1275 1.2558
175 1.32493 550 1.30121 925 1.27666 1300 1.25441
200 1.32368 575 1.29951 950 1.2751 1325 1.25304
225 1.32235 600 1.29783 975 1.27355 1350 1.25167
250 1.32093 625 1.29615 1000 1.272 1375 1.25033
275 1.31945 650 1.29447 1025 1.27047 1400 1.249
300 1.31792 675 1.29281 1050 1.26895 1425 1.24769
325 1.31633 700 1.29116 1075 1.26744 1450 1.24639
350 1.31471 725 1.28951 1100 1.26594 1475 1.24511
375 1.31306 750 1.28787 1125 1.26445 1500 1.24385
400 1.31139 775 1.28624 1150 1.26298
425 1.30971 800 1.28462 1175 1.26151
©Smar Research Corporation 16 MFR-0902
Mass Flow Rate - Appendix B
The following graph plots isentropic exponent of steam versus temperature. A linear
trend line has been added to provide an equation for the value of k at any T. A linear
function was chosen based on its simplicity and its accuracy to Figure 7 of the ASME
Steam Tables.
Isentropic Exponent of Steam
1.34
1.33
1.32
1.31
Isentropic Exponent (k)
1.3
1.29
1.28
1.27
1.26
k = -6.24543E-05T + 1.33544
1.25
1.24
1.23
0 200 400 600 800 1000 1200 1400 1600
Temperature (F)
©Smar Research Corporation 17 MFR-0902
Mass Flow Rate - Appendix C
Appendix C
Formulation of Steam Viscosity Approximation
According to the ASME Steam Tables, steam viscosity is a function of both tem-
perature and pressure. Its value can be found by applying temperature and pressure
to Table 8 of the ASME Steam Tables. Various software was also analyzed to deter-
mine what assumptions could be made.
After studying Table 8, it can be assumed that viscosity does not vary with
changing pressure. To determine an equation for viscosity as strictly a function of tem-
perature, various values of temperature were inputted into the software, while main-
taining a constant pressure. The corresponding values for viscosity were then graphed
and a trend line was formulated. The equation of this trend line would provide values
for viscosity for all possible values of temperature.
The following table is the values of steam viscosity provided by the software at
various temperatures and constant pressure:
Temp Vis(10-6 lb/ Temp Vis(10-6 lb/ Temp Vis(10-6 lb/
ft-s) ft-s)13.7 ft-s)
125 7.2 550 13.3 975 19.9
150 7.5 575 13.7 1000 20.2
175 7.8 600 14.1 1025 20.6
200 8.1 625 14.4 1050 21
225 8.5 650 14.8 1075 21.4
250 8.8 675 15.2 1100 21.7
275 9.2 700 15.6 1125 22.1
300 9.5 725 16 1150 22.5
325 9.9 750 16.4 1175 22.9
350 10.2 775 16.8 1200 23.2
375 10.6 800 17.2 1250 24
400 11 825 17.6 1300 24.7
425 11.4 850 17.9 1350 25.4
450 11.7 875 18.3 1400 26.1
475 12.1 900 18.7 1450 26.8
500 12.5 925 19.1 1500 27.5
525 12.9 950 19.5
©Smar Research Corporation 18 MFR-0902
Mass Flow Rate - Appendix C
The following graph plots steam viscosity versus temperature. A linear trend line has
been added to provide an equation for the value of m at any T. A linear function was
chosen based on its simplicity and its accuracy to Table 8 of the ASME Steam Tables.
Steam Viscosity
30
25
20
Viscosity (10^-6 lb/ft-s)
-6
Viscosity = (0.01511T + 5.05093)*10
15
10
0
0 200 400 600 800 1000 1200 1400 1600
Temperature (F)
©Smar Research Corporation 19 MFR-0902
Mass Flow Rate - Appendix D
Appendix D
Custom Liquid Calculations
If a liquid is used that is not listed in the database, then the following information
must be provided in order to complete the necessary calculations:
Critical Temperature (Tc)
Viscosity (µ1) at Temperature (T1)
Density (ρb) at 20 °C (68 °F)
Use the following equation to solve for liquid density:
1.5
Tf T1 0.9 Tc
µf µ1
T1 Tf 0.9 Tc
The following method for solving liquid viscosity is derived from Figure 2.19 of the
Flow Measurement Engineering Handbook:
Solve for Y:
Y log µ 1 1
Solve for T:
15 6 12 5 11 4 7 3 4 2 2
4.4 x10 T 3.84 x10 T 3.37 x10 T 9.23 x10 T 3.66 x10 T 6.46 x10 T 6 Y 0
Calculate T1:
T1 = T + Tf - T1
Solve for Y1 :
1 15 1 6 12 1 5 11 1 4 7 1 3 4 1 2 2 1
Y 4.4 x10 T 3.84 x10 T 3.37 x10 T 9.23 x10 T 3.66 x10 T 6.46 x10 T 6
Solve for µf:
1 1
µf log Y 1
©Smar Research Corporation 20 MFR-0902
Mass Flow Rate - Appendix D
Appendix D
Custom Gas Calculations
If a gas is used that is not listed in the database, then the following information
must be provided in order to complete the necessary calculations:
Critical Temperature (Tc)
Isentropic Exponent (k)
Molecular Weight (Mw )
Viscosity (µ1) at Temperature (T1)
Compressibility (Z)
Use the provided isentropic exponent along with the equations from Section VI
to solve for the expansion factor. Use provided molecular weight and compressibility
along with the gas equation from Section VIII to solve for the gas density. Use the fol-
lowing equation to solve for the gas viscosity:
1.5
Tf T1 0.9 Tc
µf µ1
T1 Tf 0.9 Tc
©Smar Research Corporation 21 MFR-0902
Mass Flow Rate - Appendix E
Appendix E
Natural Gas Compressibility Equations
There are three methods that can be used to solve for the compressibility factor
of natural gas. The detail characterization method requires that the mole fraction of all
elements of the natural gas be known. The gross characterization method has two op-
tions. One option requires that the real gas relative density, volumetric gross heating
value, and the mole fraction of carbon dioxide must be known; while the other option
requires that the real gas relative density, mole fraction of carbon dioxide, and mole
fraction of nitrogen must be known. Once a method is chosen use the corresponding
equations to solve for the compressibility of natural gas:
Detail Characterization Method
18 58
D.B u u k b k
D . C'n T
n n n n n
Z 1 C'n T bn cn kn D D exp cn D
3
K n = 13 n = 13
Z = compressibility factor
B = second virial coefficient
C`n = coefficients which are functions of composition
D = reduced density
K = mixture size parameter
T = absolute temperature
bn, cn, kn, un = constants given in Table 4 (AGA Report 8)
2
N 5 N 1 N 5
5 2 5 2
K = xi Ki 2 xi xj Kij 1 Ki Kj
i= 1 i= 1 j= i 1
xi = mole fraction of ith component
Ki = size parameter of ith component (Table 5)
Kij = binary interaction parameter for size (Table 6)
N = number of components in the gas mixture
18 N N 3
u u
n. n 2
B an T xi xj Eij Ki Kj B'nij
n=1 i= 1 j= 1
an = constant given in Table 4
Eij = second virial coefficient binary energy parameter
B`nij = binary characterization coefficient
1
2
Eij E'ij Ei Ej
Ei = characteristic energy parameter for ith component (Table 5)
E`ij = second virial coefficient energy binary interaction parameter (Table 6)
©Smar Research Corporation 22 MFR-0902
Mass Flow Rate - Appendix E
f
n
1 1
g q s w
n n 2 2 n n
B'nij Gij 1 gn Qi Qj 1 qn Fi Fj 1 fn Si Sj 1 sn Wi Wj 1 wn
Gij = binary orientation parameter
Qi = quadrupole parameter for ith component (Table 5)
Fi = high temperature parameter for ith component (Table 5)
Si = dipole parameter for ith component (Table 5)
Wi = association parameter for ith component (Table 5)
gn, qn, fn, sn, wn = constants given in Table 4
G'ij Gi Gj
Gij
2
Gi = orientation parameter for ith component (Table 5)
G`ij = binary interaction parameter for orientation (Table 6)
q
g n f u
n 2 n n
C'n an G 1 gn Q 1 qn F 1 fn U
G = orientation parameter
Q = quadrupole parameter
F = mixture high temperature parameter
U = mixture energy parameter
N N 1 N
G xi Gi xi xj G'ij 1 Gi Gj
i= 1 i= 1 j= i 1
N
Q xi Qi
i= 1
N
2
F xi Fi
i= 1
2
N 5 N 1 N 5
2 5 2
U xi Ei 2 xi xj Uij 1 Ei Ej
i= 1 i= 1 j= i 1
Uij = binary interaction parameter for conformal energy (Table 6)
3
D K d
d = molar density (moles per unit volume)
Solve for d using following equation:
18 58 k
n
u u k c D
n n n b n
P dRT 1 Bd D C'n T C'n T bn cn kn D D e
n = 13 n = 13
©Smar Research Corporation 23 MFR-0902
Mass Flow Rate - Appendix E
Gross Characterization Method
2
Z 1 Bmix d Cmix d
Z = compressibility factor
Bmix = second virial coefficient for the mixture
Cmix = third virial coefficient for the mixture
d = molar density (moles per unit volume)
N N
Bmix Bij xi xj
i= 1 j= 1
Bij = individual component interaction second virial coefficient
N = number of components in gas mixture
xi , xj , xk = mole fractions of gas components
N N N
Cmix Cijk xi xj xk
i= 1 j= 1 k= 1
Cijk = individual component interaction third virial coefficient
Expansions of B mix and C mix are provided on page 30 of AGA Report 8.
2
Bij b0 b1 T b2 T
b0, b1, b2 = constants given in Table 7
T = temperature
2
Cijk c0 ci T c2 T
c 0, c1, c2 = constants given in Table 7
2
BCH CH
B0 B1 HCH B2 HCH
2
CCH CH CH
C0 C1 HCH C2 HCH
HCH = molar gross heating value of the equivalent hydrocarbon
2
Bi b i0 b i1 T b i2 T
2
Ci ci0 ci1 T ci2 T
i = 0, 1, 2
bi0 , bi1 , bi2 , ci0 , c i1 , ci2 = constants given in Table 8
©Smar Research Corporation 24 MFR-0902
Mass Flow Rate - Appendix E
5 2
BN2 N2
BCH CH
BN2 CH
0.72 1.875 x10 ( 320 T)
2
1
2
BCO2 CH
0.865 BCO2 CO2
BCH CH
1
3
2
CN2 CH CH
( 0.92 0.0013 ( T 270 ) ) CCH CH CH
CN2 N2 N2
1
3
2
CN2 N2 CH
( 0.92 0.0013 ( T 270 ) ) CN2 N2 N2
CCH CH CH
1
3
2
CCO2 CH CH
0.92 CCH CH CH
CCO2 CO2 CO2
1
3
2
CCO2 CO2 CH
0.92 CCO2 CO2 CO2
CCH CH CH
1
3
CCO2 N2 CH
1.10 CC02 CO2 CO2
CN2 N2 N2
CCH CH CH
After Bmix and Cmix are calculated, use following equation to solve for d:
2
P dRT 1 Bmix d Cmix d
P = absolute pressure
R = gas constant
Use one of the following methods to solve for H CH
©Smar Research Corporation 25 MFR-0902
Mass Flow Rate - Appendix E
Method #1 (for determination of H CH)
Necessary input:
HV = volumetric gross heating value at reference conditions Th , Td, Pd
Gr = relative density (specific gravity) of mixture
xCO2 = mole fraction of carbon dioxide
HV. Z . R. Td 1
0 4
0
1.027 x10 Th 298.15
HN
Pd
HN0 = molar ideal gross heating value at 298.15K and 0.101325 MPa
Pd = reference pressure for molar density
R = gas constant, 8.31451 J/mol-K
Td = reference temperature for molar density
Th = reference temperature for heating value
Z0 = compressibility factor at reference conditions (set Z0=1 for initial iteration)
Gr Z R. Tgr ρ
0 0
air
Mr
Pgr
Mr = molar mass (molecular weight) of the mixture
Gr = relative density at reference conditions Tgr, Pgr
Pgr = reference pressure for relative density
Tgr = reference temperature for relative density
(ρ0)air = mass density of air at reference conditions Tgr, Pgr
0 M r ( air )
ρ Tgr , Pgr
air
R. Tgr
Bair Tgr
Pgr
Mr(air) = molar mass of air, 28.96256 g/mol
4 7 2
Bair Tgr .012527 5.91 x10 Tgr 6.62 x10 Tgr
0
Mr G2 HN M rN2 xCO2 M rN2 xCO2 M rCO2
xCH
G1 M rN2
xCH = mole fraction of equivalent hydrocarbon
G1 = -2.709328
G2 = 0.021062199
MrN2 = molar mass of nitrogen
MrCO2 = molar mass of carbon dioxide
xN2 1 xCH xCO2
xN2 = mole fraction of nitrogen
©Smar Research Corporation 26 MFR-0902
Mass Flow Rate - Appendix E
0
HN
HCH
xCH
HCH = molar gross heating value of the equivalent hydrocarbon
0
Bmix Pgr
Z 1
new
R. Tgr
(Z0) new = compressibility factor for next iteration
Bmix = second virial coefficient of mixture (calculated from previous equations)
Repeat process, continuously replacing Z0 with (Z0) new, until (Z0/Z0new-1) is less than
the convergence criteria (5x10-11 in double precision or 5x10 -7 in single precision)
©Smar Research Corporation 27 MFR-0902
Mass Flow Rate - Appendix E
Method #2 (for determination of H CH)
Necessary input:
Gr = relative density (specific gravity) of mixture
xCO2 = mole fraction of carbon dioxide
xN2 = mole fraction of nitrogen
xCH 1 xN2 xCO2
xCH = mole fraction of equivalent hydrocarbon
Gr Z R. Tgr ρ
0 0
air
Mr
Pgr
Mr = molar mass (molecular weight) of the mixture
Gr = relative density at reference conditions Tgr, Pgr
Pgr = reference pressure for relative density
R = gas constant, 8.31451 J/mol-K
Tgr = reference temperature for relative density
Z0 = compressibility factor at reference conditions (set Z0=1 for initial iteration)
(ρ0)air = mass density of air at reference conditions Tgr, Pgr
0 M r ( air )
ρ Tgr , Pgr
air
R. Tgr
Bair Tgr
Pgr
Mr(air) = molar mass of air, 28.96256 g/mol
4 7 2
Bair Tgr .012527 5.91 x10 Tgr 6.62 x10 Tgr
Mr xCO2 M rCO2 xN2 M rN2
M rCH
xCH
MrCH = molar mass of equivalent hydrocarbon
MrN2 = molar mass of nitrogen
MrCO2 = molar mass of carbon dioxide
Mr = molar mass of mixture
©Smar Research Corporation 28 MFR-0902
Mass Flow Rate - Appendix E
M rCH G1
HCH
G2
HCH = molar gross heating value of the equivalent hydrocarbon
G1 = -2.709328
G2 = 0.021062199
0
Bmix Pgr
Z 1
new
R. Tgr
(Z0) new = compressibility factor for next iteration
Bmix = second virial coefficient of mixture (calculated from previous equations)
Repeat process, continuously replacing Z0 with (Z0) new, until (Z0/Z0new-1) is less than
the convergence criteria (5x10-11 in double precision or 5x10 -7 in single precision)
©Smar Research Corporation 29 MFR-0902
Mass Flow Rate - Appendix E
Compressibility Symbols and Definitions
Symbol Definition
B Second virial coefficient
Bij Individual component interaction second virial coefficient
Bmix Second virial coefficient for the mixture
B`nij Binary characterization coefficient
Cijk Individual component interaction third virial coefficient
Cmix Third virial coefficient for the mixture
C`n Coefficient as a function of composition
d Molar density (moles per unit volume)
D Reduced density
Ei Characteristic energy parameter for ith component (Table 5)
Eij Second virial coefficient binary energy parameter
E`ij Second virial coefficient binary interaction parameter (Table 6)
F Mixture high temperature parameter
Fi High temperature parameter for ith component (Table 5)
G Orientation parameter
Gi Orientation parameter for ith component (Table 5)
Gij Binary orientation parameter
G`ij Binary interaction parameter for orientation (Table 6)
Gr Relative density (specific value) of mixture
HCH Molar gross heating value of the equivalent hydrocarbon
HN0 Molar ideal gross heating value at 298.15K and 0.101325 MPa
HV Volumetric gross heating value at reference conditions Th, Td, Pd
K Mixture size parameter
Ki Size parameter of ith component (Table 5)
Kij Binary interaction parameter for size (Table 6)
Mr Molar mass (molecular weight) of the mixture
Mr(air) Molar mass of air, 28.96256 g/mol
Mri Molar mass of ith component
N Number of components in the gas mixture
P Absolute pressure
Pd Reference pressure for molar density
Pgr Reference pressure for relative density
Q Quadrupole parameter
Qi Quadrupole parameter for ith component (Table 5)
R Gas constant, 8.31451 J/mol-K
r0air Mass density of air at reference conditions Tgr, Pgr
Si Dipole parameter for ith component (Table 5)
T Absolute temperature
Td Reference temperature for molar density
Tgr Reference temperature for relative density
Th Reference temperature for heating value
U Mixture energy parameter
Uij Binary interaction parameter for conformal energy (Table 6)
Wi Association parameter for ith component (Table 5)
xi Mole fraction of ith component
Z Compressibility factor
Z0 Compressibility factor at reference conditions
Z0new Compressibility factor for next iteration
an, bn, c n, fn, gn, k n, qn, sn, un, wn Constants given in Table 4
b0, b1, b2, c0, c1, c 2 Constants given in Table 7
bi0, bi1, bi2, c i0, c i1, ci2 Constants given in Table 8
©Smar Research Corporation 30 MFR-0902
Mass Flow Rate - Appendix F
Appendix F
Iterative Process Used to Solve for Coefficient of Discharge
1) Set Re equal to ∞ and solve for C d
2) Multiply this value for C d by the invariant A 1 to obtain new value of Re:
Ev Yd . 2 ρ f . ∆ P
2
A1
µf D
3) Use new value of Re to solve for new value of C d
4) Repeat process until:
Re
A1
Cd
1 . 10
4
A1
For example:
If a long radius nozzle (ISO) had values:
A1 = 100,000
β = 0.5
6 0.5
.5 10
Cd 0.9965 0.00653 . β .
Re
1) at Re = ∞:
Cd = 0.9965
2) C d * A 1 = 99,650 2) C d(1) * A 1 = 98,190
Re(1) = 99,650 Re(2) = 98,190
3) at Re (1) = 99,650 3) at Re (2) = 98,190
Cd(1) = .9819 Cd(2) = .9818
4) (A 1 – (Re/C d))/A 1 > 1* 10 -4 4) (A 1 – (Re/C d))/A 1 = 1 * 10-4
Repeat from step 2 Therefore, C d = .9818
©Smar Research Corporation 31 MFR-0902
Mass Flow Rate - Appendix G
Appendix G
References
AGA 3, Orifice Metering of Natural Gas and Other Related Hydrocarbons, Part 1:
General Equations and Uncertainty Guidelines, 3rd ed., American Gas
Association, AGA Catalog No. XQ9210, Arlington, VA., 1990.
AGA 3, Orifice Metering of Natural Gas and Other Related Hydrocarbons, Part 2:
Specification and Installation Requirements, 4th ed., American Gas Association,
AGA Catalog No. XQ0002, Arlington, VA., 2000.
AGA 3, Orifice Metering of Natural Gas and Other Related Hydrocarbons, Part 3:
Natural Gas Applications, 3rd ed., American Gas Association,
AGA Catalog No. XQ9210, Arlington, VA., 1992.
AGA 3, Orifice Metering of Natural Gas and Other Related Hydrocarbons, Part :
Background, Development, Implementation Procedure, and Subroutine
Documentation for Empirical Flange-Tapped Discharge Coefficient Equation,
3rd ed., American Gas Association, AGA Catalog No. XQ9211, Arlington, VA.,
1992.
AGA 8, Compressibility Factors of Natural Gas and Other Related Hydrocarbon
Gases, Transmission Measurement Committee Report No. 8, AGA Catalog No.
XQ 9212, Arlington, VA., 1992.
ASME: ASME International Steam Tables for Industrial Use, American Society of
Mechanical Engineers, New York, 2000.
ASME Standard MFC-14M-2001, Measurement of Fluid Flow Using Small Bore
Precision Orifice Meters, American Society of Mechanical Engineers, New York,
2001.
ASME Standard MFC-3M-1989, Measurement of Fluid Flow in Pipes Using Orifice,
Nozzle, and Venturi, American Society of Mechanical Engineers, New York,
1989.
GPA Standard 2145-00, Table of Physical Constants for Hydrocarbons and Other
Compounds of Interest to the Natural Gas Industry, Gas Producers Association,
Tulsa, OK., 2000.
ISO Standard 5167-1, Measurement of Fluid Flow by Means of Pressure Differential
Devices, International Standards Organization, Geneva, 1991.
Miller, R. W.: Flow Measurement Engineering Handbook, 3rd ed., McGraw-Hill, New
York, 1996.
©Smar Research Corporation 32 MFR-0902
Smar Research reserves the right to make changes to design and functionality of any product without notice. Smar Research
does not assume any liability arising out of the application or use of any product. Smar Research , Technology Source, and the
SRC logo are registered trademarks of Smar Research Corporation. The HART, Fieldbus, and Profibus Foundation logos are
trademarks of their respective owners.
Smar Research Corporation
4250 Veterans Memorial Highway
Holbrook, NY USA 11741
Tel: 631.737.3111 Fax: 631.737.3892
techinfo@SmarResearch.com
www.SmarResearch.com
©Smar Research Corporation 33 MFR-0902
You might also like
- Proper Air-Fuel Ratios for Starting, Idling, Accelerating & MoreDocument9 pagesProper Air-Fuel Ratios for Starting, Idling, Accelerating & MoreVinh Do ThanhNo ratings yet
- Air-Fuel Ratio, Lambda and Engine Performance: AFR M MDocument12 pagesAir-Fuel Ratio, Lambda and Engine Performance: AFR M MVinh Do ThanhNo ratings yet
- Studies On Drying Kinetics of Solids in A Rotary DryerDocument6 pagesStudies On Drying Kinetics of Solids in A Rotary DryerVinh Do ThanhNo ratings yet
- NPK-15 8 15Document5 pagesNPK-15 8 15Vinh Do ThanhNo ratings yet
- Multi-Use Chair DesignDocument7 pagesMulti-Use Chair DesignVinh Do ThanhNo ratings yet
- Modeling and Simulation of A Co-Current Rotary Dryer Under Steady ConditionsDocument8 pagesModeling and Simulation of A Co-Current Rotary Dryer Under Steady ConditionsVinh Do ThanhNo ratings yet
- Modelling and Simulation of A Direct Contact Rotary DryerDocument16 pagesModelling and Simulation of A Direct Contact Rotary DryerVinh Do ThanhNo ratings yet
- Ansi B16-104Document1 pageAnsi B16-104Monica Suarez100% (1)
- Effects of Drying Parameters On Heat Transfer During DryingDocument13 pagesEffects of Drying Parameters On Heat Transfer During DryingVinh Do ThanhNo ratings yet
- Dryer CalculationsDocument4 pagesDryer CalculationsVinh Do Thanh0% (1)
- The Heart of Operations - World Cement - 02-2015Document4 pagesThe Heart of Operations - World Cement - 02-2015fetniNo ratings yet
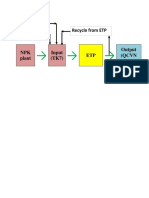
- Recycle From ETP Make Up H2O DAP, UreaDocument1 pageRecycle From ETP Make Up H2O DAP, UreaVinh Do ThanhNo ratings yet
- Equivalent Grades of Cast IronsDocument2 pagesEquivalent Grades of Cast IronsVinh Do ThanhNo ratings yet
- 4244 12672 1 PB PDFDocument15 pages4244 12672 1 PB PDFVinh Do ThanhNo ratings yet
- Aoac - Methods.1.1990. MoistureDocument2 pagesAoac - Methods.1.1990. MoistureVinh Do ThanhNo ratings yet
- DRS 279-2015 Organic Fertilizer - SpecificationDocument17 pagesDRS 279-2015 Organic Fertilizer - SpecificationVinh Do ThanhNo ratings yet
- PEP Report 267A: Ihs ChemicalDocument8 pagesPEP Report 267A: Ihs ChemicalVinh Do ThanhNo ratings yet
- 4244 12672 1 PB PDFDocument15 pages4244 12672 1 PB PDFVinh Do ThanhNo ratings yet
- Mau Giay Uy Quyen Bang Tieng AnhDocument3 pagesMau Giay Uy Quyen Bang Tieng AnhVinh Do ThanhNo ratings yet
- Natural Evaporation RateDocument16 pagesNatural Evaporation RateVinh Do ThanhNo ratings yet
- Metal Price IndexDocument1 pageMetal Price IndexVinh Do ThanhNo ratings yet
- Tinh Luong Nuoc Bay HoiDocument22 pagesTinh Luong Nuoc Bay HoiVinh Do ThanhNo ratings yet
- Review On Development of Polypropylene Manufacturing ProcessDocument11 pagesReview On Development of Polypropylene Manufacturing ProcessShweta Yadav100% (1)
- Estimating Evaporation From Water SurfacesDocument27 pagesEstimating Evaporation From Water SurfacesVinh Do ThanhNo ratings yet
- CRACKER A PC Based Simulator For Industr PDFDocument6 pagesCRACKER A PC Based Simulator For Industr PDFVinh Do ThanhNo ratings yet
- How To Calculate Heat Load - 5 StepsDocument1 pageHow To Calculate Heat Load - 5 StepsVinh Do ThanhNo ratings yet
- 1 0ProjectManagementProceduresDocument8 pages1 0ProjectManagementProceduresRamiesRahmanNo ratings yet
- Optimization of Wall Thickness For Minimum Heat LossesDocument9 pagesOptimization of Wall Thickness For Minimum Heat LossesVinh Do ThanhNo ratings yet
- How To Calculate Heat Load - 5 StepsDocument1 pageHow To Calculate Heat Load - 5 StepsVinh Do ThanhNo ratings yet
- Investigation of Sensible Heat Storage and Heat Insulation in The Exploitation of Concentrated Solar EnergyDocument5 pagesInvestigation of Sensible Heat Storage and Heat Insulation in The Exploitation of Concentrated Solar EnergyradanpetricaNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Pressure-Sensitive Adhesives and Applications, Second 2ed - 0824750594 PDFDocument761 pagesPressure-Sensitive Adhesives and Applications, Second 2ed - 0824750594 PDFPatricia CantieriNo ratings yet
- Astm 2196Document5 pagesAstm 2196Alexandre LiraNo ratings yet
- 16 Marks Quesrtion Bank Without Unit 1Document8 pages16 Marks Quesrtion Bank Without Unit 1mohanNo ratings yet
- The Numerical Simulation of Two Phase Flow in Settling Tanks DanielBrennanPhDDocument127 pagesThe Numerical Simulation of Two Phase Flow in Settling Tanks DanielBrennanPhDAghajani100% (1)
- Cooling Tower Fill EfficiencyDocument11 pagesCooling Tower Fill EfficiencyMubarik Ali100% (1)
- Report - On The Identification of A VortexDocument7 pagesReport - On The Identification of A VortexPiyush JagasiaNo ratings yet
- Gomez Et Al Unified Mechanistic Model For Steady-State PDFDocument12 pagesGomez Et Al Unified Mechanistic Model For Steady-State PDFFrancisco OppsNo ratings yet
- HexaneDocument3 pagesHexaneIan RidzuanNo ratings yet
- SEC1.pdf MMADocument67 pagesSEC1.pdf MMARicardoNo ratings yet
- Lecture 1Document58 pagesLecture 1Suara Kabir AdewaleNo ratings yet
- Teikoku Canned Motor Pumps: Sealless Design for Zero LeakageDocument16 pagesTeikoku Canned Motor Pumps: Sealless Design for Zero LeakageHideyoshi Ardi ToyotomiNo ratings yet
- Sucker Rod String Design by LufkinDocument9 pagesSucker Rod String Design by Lufkinachmad mahmudyNo ratings yet
- Final FM 2019Document102 pagesFinal FM 2019asad aliNo ratings yet
- Single Counterbalance, Relief Compensated: A-VBSO-SE-CC-30-PL 08.45.17 - X - Y - ZDocument2 pagesSingle Counterbalance, Relief Compensated: A-VBSO-SE-CC-30-PL 08.45.17 - X - Y - Znemi90No ratings yet
- Mechanical MeasurementsDocument13 pagesMechanical MeasurementscaptainhassNo ratings yet
- Covection Heat TransferDocument24 pagesCovection Heat TransfersampathsiddamNo ratings yet
- Heat Transfer Characteristics in Nanofluid-A ReviewDocument10 pagesHeat Transfer Characteristics in Nanofluid-A ReviewTatiana CaballeroNo ratings yet
- AppendixDocument11 pagesAppendixVishal PatadiaNo ratings yet
- Flow of Incompressible Fluids in Conduits and ThinDocument81 pagesFlow of Incompressible Fluids in Conduits and Thinkruthi_dhoriaNo ratings yet
- Air To Boil TemperatureDocument8 pagesAir To Boil TemperatureRafa Lopez PuigdollersNo ratings yet
- A Comparison of Liquid Petroleum Meters For Custody Transfer MeasurementDocument12 pagesA Comparison of Liquid Petroleum Meters For Custody Transfer MeasurementAmr Guenena100% (2)
- Mixing eHANDBOOK: Master Your Mixing - Select the Right BlenderDocument18 pagesMixing eHANDBOOK: Master Your Mixing - Select the Right BlenderMaicon GoularteNo ratings yet
- Separador TrifásicoDocument8 pagesSeparador TrifásicoPROCESOS PROCESOSNo ratings yet
- On Heat and Mass Transfer in The Unsteady Squeezing Flow Between Parallel PlatesDocument9 pagesOn Heat and Mass Transfer in The Unsteady Squeezing Flow Between Parallel PlatesHasan AhmedNo ratings yet
- Daikin DAIFLOILDocument2 pagesDaikin DAIFLOILZaw Khaing WinNo ratings yet
- Fill Fluid PDFDocument10 pagesFill Fluid PDFGalih YugaNo ratings yet
- Viscosity Conversion Chart GuideDocument3 pagesViscosity Conversion Chart GuideKhafid Al Na'imNo ratings yet
- Shell Tellus s2 M 46Document3 pagesShell Tellus s2 M 46Azad KiyalNo ratings yet
- VANZAN Brochure English Web PDFDocument18 pagesVANZAN Brochure English Web PDFMarielaIkutaNo ratings yet
- The Major Characteristics of AdhesivesDocument9 pagesThe Major Characteristics of Adhesivesrodrigo_venancioNo ratings yet