Professional Documents
Culture Documents
Caracterizacion y Neutralizacion de Toxinas y Cia de ETEC
Uploaded by
Jesus Daniel Rojas RiveroOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Caracterizacion y Neutralizacion de Toxinas y Cia de ETEC
Uploaded by
Jesus Daniel Rojas RiveroCopyright:
Available Formats
CLINICAL AND VACCINE IMMUNOLOGY, Dec. 2010, p. 18591867 1556-6811/10/$12.00 doi:10.1128/CVI.00251-10 Copyright 2010, American Society for Microbiology.
. All Rights Reserved.
Vol. 17, No. 12
Escherichia coli K88ac Fimbriae Expressing Heat-Labile and Heat-Stable (STa) Toxin Epitopes Elicit Antibodies That Neutralize Cholera Toxin and STa Toxin and Inhibit Adherence of K88ac Fimbrial E. coli
Chengxian Zhang and Weiping Zhang*
Veterinary and Biomedical Sciences Department, The Center for Infectious Disease Research and Vaccinology, South Dakota State University, Brookings, South Dakota 57007
Received 18 June 2010/Returned for modication 28 July 2010/Accepted 14 October 2010
Enterotoxigenic Escherichia coli (ETEC) strains are a major cause of diarrheal disease in humans and animals. Bacterial adhesins and heat-labile (LT) and heat-stable (ST) enterotoxins are the virulence determinants in ETEC diarrhea. It is believed that vaccines inducing anti-adhesin immunity to inhibit bacterial adherence and anti-toxin immunity to eliminate toxin activity would provide broad-spectrum protection against ETEC. In this study, an ETEC mbrial adhesin was used as a platform to express LT and STa for adhesin-toxin fusion antigens to induce anti-toxin and anti-adhesin immunity. An epitope from the B subunit of LT toxin (LTP1, 8LCSEYRNTQIYTIN21) and an STa toxoid epitope (5CCELCCNPQCAGCY18) were embedded in the FaeG major subunit of E. coli K88ac mbriae. Constructed K88ac-toxin chimeric mbriae were harvested and used for rabbit immunization. Immunized rabbits developed anti-K88ac, anti-LT, and anti-STa antibodies. Moreover, induced antibodies not only inhibited adherence of K88ac mbrial E. coli to porcine small intestinal enterocytes but also neutralized cholera toxin and STa toxin. Data from this study demonstrated that K88ac mbriae expressing LT and STa epitope antigens elicited neutralizing anti-toxin antibodies and anti-adhesin antibodies and suggested that E. coli mbriae could serve as a platform for the development of broad-spectrum vaccines against ETEC. Enterotoxigenic Escherichia coli (ETEC) strains that colonize the host small intestine and produce heat-labile (LT) and/or heat-stable (ST) enterotoxins are a major cause of diarrheal disease in humans and farm animals. The virulence determinants of ETEC in diarrhea are bacterial adhesins and enterotoxins (1, 7, 24, 25, 37, 41, 43). Adhesins mediate the attachment of ETEC bacteria to host epithelium cells in the small intestine and facilitate subsequent bacterial colonization. Enterotoxins, including ST and LT toxins (17, 18, 33), disrupt host uid homeostasis and cause uid and electrolyte hypersecretion through activation of adenyl cyclase (by LT) or guanylate cyclase (by STa) in small intestinal epithelial cells (19, 23). Recent experimental studies using a porcine model demonstrated that ETEC strains expressing LT or STa as the only toxin are sufciently virulent to cause diarrhea (7, 43, 46). No vaccines are currently available to effectively protect humans and animals against ETEC infections. Experimental oral vaccines carrying adhesin antigens alone showed protection against colonization by ETEC strains expressing the same or homologous adhesins (40). Similarly, experimental antitoxin vaccines using toxin antigens, mainly LT toxoids or LTB subunits, could not provide effective protection either. Evidence indicated that LT antigen-based experimental vaccines provided protection against only LT-producing ETEC strains but not against ETEC strains that produce STa toxin (13, 14). As more than two-thirds of human ETEC diarrheal cases and more than one-quarter of porcine ETEC diarrhea cases are caused by STa-producing ETEC strains (15, 16, 28, 31, 35, 42, 48), anti-toxin vaccines must also induce anti-STa immunity in order to provide effective protection against ETEC toxins. It becomes evident that both anti-toxin immunity, including anti-LT and anti-ST immunity, and anti-adhesin immunity are needed for broadly effective protection against ETEC-associated diarrhea (36). Anti-toxin immunity and anti-adhesin immunity can be simultaneously induced by adhesin-toxin chimeric antigens. When an LT or an LTB subunit was fused to a CFA/I or a CS adhesin, the resultant chimera elicited both anti-LT and antiadhesin immunity (20, 22, 30). Similarly, chimeric mbriae with an STa peptide expressed in ETEC adhesin CS31A elicited neutralizing anti-STa antibodies (4, 5). However, no adhesin-toxin chimeric antigens have been constructed for stimulation of both anti-LT and anti-STa anti-toxin immunity. Expressing both LT and STa toxin antigens in one adhesin could produce a single chimeric antigen to induce not only anti-adhesin immunity but also anti-LT and anti-STa immunity. Moreover, as E. coli adhesins bind to host receptors in the small intestine, such adhesin-toxin chimeric antigens, we believe, could have an advantage in directly inducing host mucosal immunity, which is believed to play a critical role in protection against enteric infections. In this study, we expressed an epitope from the LTB subunit designated LTP1 and an epitope from an STa toxoid at the FaeG major subunit of E. coli K88ac mbriae and examined the ability of these K88ac-toxin mbrial antigens to induce both anti-adhesin immunity and anti-toxin (anti-LT and antiSTa) immunity. This LTP1 epitope is homologous to an
1859
* Corresponding author. Mailing address: Veterinary and Biomedical Sciences Department, The Center for Infectious Disease Research and Vaccinology, South Dakota State University, Box 2157, North Campus Drive, Brookings, SD 57006. Phone: (605) 688-4317. Fax: (605) 688-6003. E-mail: weiping.zhang@sdstate.edu. Published ahead of print on 27 October 2010.
1860
ZHANG AND ZHANG
CLIN. VACCINE IMMUNOL.
TABLE 1. PCR primers used to construct chimeric faeG major subunit genes of K88ac mbriaea
Primer Sequence (5 3 )
Eco81IG-F ...............................................................GTG AGG ATG TGG TGA TTA CCG TGC CTG AGG C SpeIG-R ...................................................................TTA TTG ATA CGT TCT GAT AAC AGA CTA GTA TC K88ac3:LTb-F..........................................................TAT CGC AAC ACA CAA ATA TAT ACG ATA AAT GGT TTA GCA TAT TTT GTT K88ac3:LTb-R .........................................................TTG TGT GTT GCG ATA TTC CGA ACA TAG TAC TAC AGA AGC TCC TTC K88ac4:LTb-F..........................................................TAT CGC AAC ACA CAA ATA TAT ACG ATA AAT AAA GTG AAT GCA TCT TAT GCC K88ac4:LTb-R .........................................................TTG TGT GTT GCG ATA TTC CGA ACA TAG CGG CAG AAC AAC AAA ATA TGC TA K88ac3:pSTa13-F ....................................................TGT TGT AAT CCT CAT TGT GCT GGA TGT TAT GGT TTA GCA TAT TTT GTT K88ac3:pSTa13-R ...................................................ACA CTG AGG ATT ACA ACA AAG TTC ACA GCA TAC TAC AGA AGC TCC TTC
a
Nucleotides underlined represent restriction enzyme sites, and nucleotides in italics are of the LTB epitope or the STa13 epitope.
epitope of the cholera toxin (CT) B subunit (CTP1; 8LAAEY HNTQIHTLD21). CT produced by Vibrio cholerae is highly homologous in function and structure to the LT toxin produced by ETEC strains, and CT is commonly used to replace LT in various assays. This CTP1 had been successfully expressed in agella of Salmonella muenchen. After being immunized with this chimeric agellum-CTP1 antigen, mice developed anti-CT antibodies (10). The STa epitope used in this study is a shorter peptide of porcine STa toxoid STa13. When its 13th amino acid was substituted, the modied STa protein was no longer toxic but showed anti-STa immunogenicity if carried by an LT toxoid protein (47). Because this LTP1 epitope was from the nontoxic LTB subunit and the STa13 epitope was from the STa13 toxoid, they are considered safe antigens. These two epitopes were expressed individually or combined at a FaeG major subunit of K88ac mbriae, and resultant chimeric K88ac mbriae were puried and used for rabbit immunization. Serum and fecal samples from the immunized rabbits were examined for anti-LT, anti-STa, and anti-adhesin antibodies, and the abilities of these antibodies to neutralize LT and STa toxins and inhibit K88ac mbrial adherence in vitro were further studied.
MATERIALS AND METHODS Bacterial strains and plasmids. E. coli strain TOP10 (Invitrogen, Carlsbad, CA) was used to construct experimental strains and as the negative control in this study. Plasmid p8069 (44), derived from plasmid pBAD88-102, which carries the entire operon expressing K88ac mbriae (3), was used as a vector in chimeric gene and mbria construction. Constructed E. coli strains that express chimeric K88ac mbriae were cultured in LB medium supplemented with ampicillin (50 g/ml). Construction of chimeric K88ac faeG genes for chimeric FaeG-toxin major subunits. From our previous study to determine the signicance of major subunit epitopes in K88ac mbrial binding activity, we identied two surface-exposed peptides from the FaeG major subunit of K88ac mbriae, EP3 (95LRNPDGET NKK105) and EP4 (114MKNAEGTKVGSV122), and discovered that the removal of either peptide did not affect the biosynthesis or binding activity of K88ac mbriae (unpublished data). Thus, EP3 and EP4 became primary targets to be replaced for foreign peptide expression. To construct chimeric faeG genes, we designed specic PCR primers (Table 1) and used a three-step PCR method to substitute nucleotides encoding the EP3 and/or the EP4 peptide with nucleotides encoding the LTP1 epitope (8LCSEYRNTQIYTIN21) and/or the STa13 toxoid peptide (5CCELCCNPQCAGCY18; underlined Q is the 13th amino acid replacing the original A residue of the porcine STa toxin). Briey, we conducted one PCR using primers Eco81I-F and an insert-R (K88ac3:LTb-R or K88ac3: pSTa13-R) to amplify the 5 -end faeG gene upstream at EP3 and a second PCR using primers of an insert-F (K88ac3:LTb-F or K88ac3:STa13-F) and SpeIG-R to amplify the 3 -end faeG gene downstream at EP3 (Fig. 1A). Since the insert-F and insert-R primers contained overlapping complementary nucleotides encoding the LTP1 epitope or the STa13 epitope, amplicons from two PCRs were able to be fused in a third splicing overlap extension (SOE) PCR to produce a chimeric faeG gene. This chimeric faeG gene had nucleotides encoding EP3
replaced with nucleotides encoding the LTP1 or the STa13 epitope. Similarly, amplied products from one PCR using Eco81I-F paired with an insert-R primer (K88ac4:LTb-R or K88ac4:STa13-R) and from a second PCR with an insert-F primer (K88ac4:LTb-F or K88ac4:STa13-F) paired with SpeIG-R were fused in a third SOE PCR to generate a chimeric faeG gene which had its nucleotides of EP4 substituted with those of the LTP1 or the STa13 epitope. In addition, we double mutated the faeG gene by replacing the EP3 nucleotides with those of the STa13 toxoid epitope and the EP4 nucleotides with those of LTP1 for a double mutant. PCRs were performed with a PTC-100 thermal cycler (Bio-Rad, Hercules, CA) in a 50- l reaction mixture containing 1 Pfu DNA polymerase buffer (with Mg2 ), 200 nM deoxynucleoside triphosphate (dNTP), forward and reverse primers at 0.5 M each, and 1 U Pfu DNA polymerase (Stratagene, La Jolla, CA). SOE PCR was performed in a reaction mixture containing 1 Pfu DNA polymerase buffer (with Mg2 ), 200 nM dNTP, 20 l each of the 5 - and 3 -end puried PCR products, 1 U of Pfu polymerase, and 0.5 U Taq DNA polymerase (Applied Biosystems, Foster City, CA). PCR-amplied products were separated by gel electrophoresis and puried using a QIAquick gel extraction kit (Qiagen, Valencia, CA). Puried PCR products of each chimeric faeG gene (insert) and plasmid p8069 (vector) were digested with the Eco81I and SpeI restriction enzymes (New England BioLabs, Ipswich, MA) and ligated with T4 DNA ligase (Invitrogen) under standard conditions (2). Ligated products were introduced into E. coli TOP10 cells by standard electroporation (2). Positive colonies selected with ampicillin were screened by PCR initially and then sequenced to ensure that the cloned chimeric genes were inserted in the correct reading frame. Expression of chimeric FaeG major subunit proteins. FaeG-toxin major subunit proteins expressed from the constructed chimeric faeG genes were examined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Each constructed strain was cultured in LB medium with ampicillin (50 g/ml) at 37C overnight. The optical densities (ODs) of overnight-grown cultures were measured, and equal amounts of cells (based on OD readings) were centrifuged at 3,000 g for 20 min. Culture pellets were used for total protein preparation using bacterial protein extraction reagent (in phosphate buffer; Pierce, Rockford, IL). Thirty-microliter volumes of total protein extracts (50 to 100 g) were used to detect chimeric FaeG major subunit proteins with hybridoma supernatant of anti-K88ac monoclonal antibodies (MAbs) 30/17 and 36/41(38, 44), rabbit anti-CT antiserum, and rabbit anti-STa antiserum, respectively. Protein extracts from strain 8069 were used as the positive control for K88ac mbriae, and extracts from strain TOP10 were used as the negative control. Total protein extracts were separated in an SDS10% polyacrylamide gel and transferred to a nitrocellulose membrane. The transferred membrane was blocked with 2% fatfree milk overnight at 4C and then incubated with anti-K88ac MAb hybridoma supernatant (1:50), anti-CT serum (Sigma, St. Louis, MO) at a dilution of 1:3,000, or 1:3,000-diluted anti-STa serum (a gift from D. C. Robertson, Kansas State University). After three washes, membranes were incubated accordingly with IRDye goat anti-mouse or anti-rabbit IgG (LI-COR, Lincoln, NE) at a dilution of 1:5,000 for 1 h. After nal washes, chimeric proteins on the membranes were detected by using LI-COR Odyssey Infrared Gel Imaging System Premium (LI-COR). Immunolabeling and transmission electron microscopy (TEM) to detect biosynthesized chimeric K88ac mbriae. A single K88ac mbria is composed of hundreds of FaeG major subunits. These FaeG major subunits were assembled into a lament structure and displayed at the E. coli cell surface. To verify the biosynthesis of K88ac mbriae of chimeric FaeG major subunits, we labeled E. coli cells with anti-K88ac, anti-LT, or anti-STa antibodies and examined them by TEM. Bacteria grown overnight on agar plates were harvested, washed, and
VOL. 17, 2010
K88ac-TOXIN FIMBRIAE INDUCE PROTECTIVE IMMUNITY
1861
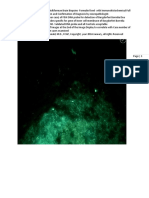
FIG. 1. Construction of chimeric faeG genes and detection of chimeric FaeG major subunits of E. coli K88ac mbriae. (A) Construction of the chimeric faeG gene with nucleotides encoding EP3 replaced with a toxin epitope. Internal forward and reverse PCR primers (insert-F and insert-R) were specically designed to replace EP3 nucleotides from the native faeG gene with nucleotides encoding the LTP1 or the STa13 peptide in a three-step PCR method. The solid black line in each insert primer represents nucleotides complementary to the faeG gene, and the gray line indicates nucleotides of an inserted epitope, including overlapping complementary nucleotides (solid). (B) Constructed single- and double-mutant strains expressing chimeric FaeG major subunits with EP3 replaced with LTP1 (strain 8551) or STa13 (strain 8616), EP4 with LTP1 (strain 8549), and EP3 with STa13 and EP4 with LTP1 (strain 8576). (C) Western blot assay for detection of chimeric FaeG proteins expressed by the constructed 8551, 8549, 8576, and 8616 strains, with strain 8069 as the K88ac FaeG-positive control and E. coli TOP10 as the negative control, by hybridoma supernatant of anti-K88ac MAbs 36/41 and 30/17, rabbit anti-CT antiserum, and rabbit anti-STa antiserum, respectively.
resuspended in phosphate-buffered saline (PBS). A 200-mesh copper grid (EMS, Hateld, PA) was incubated with a bacterial suspension of each constructed strain by oating a grid on top of a drop of a diluted bacterial suspension (1 106 to 1 107 CFU/ml) for 30 to 60 min. Each bacterium-coated grid was separately rinsed in PBST (PBS with 2% BSA and 0.05% Tween 20) three times and then individually incubated with hybridoma supernatants of anti-K88ac MAbs 36/41 and 30/17, rabbit anti-CT serum, or rabbit anti-STa serum for 30 min. After three washes with PBST and another three washes with PBS, each grid was incubated accordingly with gold-conjugated (20-nm particles) goat-antimouse or goat-anti-rabbit IgG for 30 min. Bacteria on grids were xed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4 (EMS), for 3 min, rinsed in distilled water, negatively stained using 2% phosphotungstic acid, and then air dried. TEM was performed using a JEOL-1210 electron microscope (JEOL Ltd., Tokyo, Japan), and immunolabeling images were examined at a magnication of 30,000. Porcine brush border bacterial adherence assay to examine chimeric mbriae in binding. Jejunal small intestine segments were collected from a K88ac receptor-positive piglet to prepare brush border vesicles as described previously (11, 34). Each constructed strain was examined for binding to K88ac receptor-positive brush border vesicles. The numbers of bacteria bound to a single brush border were recorded, and a total of 20 randomly selected brush border vesicles were examined for each bacterial strain. Chimeric K88ac mbria preparation and rabbit immunization. Chimeric K88ac mbriae of each constructed strain were physically harvested by following the protocol previously reported (11). Briey, each mutant strain was grown in 5 liters of tryptone soya broth medium overnight at 37C. Culture growth was centrifuged at 7,000 rpm for 20 min at 4C, and each pellet was resuspended in 50 ml PBS in a water bath at 65C for 40 min. While still warm, the suspension was blended in a tissue blender for 2 to 3 min and centrifuged at 10,000 rpm for 25 min at 4C to remove the bacterial debris (pellets) and to collect the mbriae (in supernatant). Collected supernatant was condensed with solid polyethylene glycol compound (Sigma) overnight at 4C. Fimbriae in the remaining supernatant were precipitated as the pH was gradually adjusted to 4.0 with 2.5% citric acid and collected by centrifugation at 14,000 rpm for 20 min at 4C. One hundred micrograms of puried chimeric K88ac mbriae from each constructed strain, in an equal volume of Freunds incomplete adjuvant (Sigma), was used to immunize two adult rabbits intramuscularly (i.m.). Two booster injections were administered at biweekly intervals. Blood and fecal samples were collected before and 14 days after immunization. Collected serum samples and fecal samples that were resuspended in fecal reconstitution buffer (10 mM Tris, 100 mM NaCl, 0.05% Tween 20, 5 mM sodium azide, pH 7.4) with the protease
inhibitor phenylmethylsulfonyl uoride (Sigma) at 0.5 mM were stored at 80C until use. Animal studies in this project complied with the Animal Welfare Act by following the 1996 National Research Council guidelines (26) and were approved and supervised by a state veterinarian and South Dakota State Universitys Institutional Animal Care and Use Committee. Anti-rabbit antibody titration. Rabbit serum and fecal samples were examined for anti-K88ac, anti-CT, and anti-STa antibodies. Puried porcine wild-type ETEC strain 3030-2 K88ac mbria (12), CT (Sigma), and STa ovalbumin conjugates were used as antigens in enzyme-linked immunosorbent assays (ELISAs) to titrate anti-K88ac, anti-LT, and anti-STa antibodies, respectively. Four hundred nanograms of K88ac mbriae was used to coat each well of a MaxiSorp ELISA plate (Nunc, Roskilde, Denmark) to titrate anti-K88ac antibodies in a standard ELISA. To titrate anti-LT antibodies, 400 ng GM1 (Sigma) was used to coat each well of a MaxiSorp plate and 40 ng CT was added to each well as described previously (43, 45, 47). For anti-STa antibody titration, we coated each well of a Costar plate (Corning Inc., Corning, NY) with 1.25 ng of STa ovalbumin conjugate (a gift from D. C. Robertson) as described previously (46, 47). Rabbit serum or fecal samples (in triplicate) were used as the primary antibodies (in a binary dilution), and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and IgA (1:5,000) were used as the secondary antibodies. The OD at 405 nm was measured after 20 min of development in peroxidase substrates (KPL, Gaithersburg, MD). The titration endpoint was determined as the reciprocal of the interpolated dilution giving an OD of 0.4 after subtraction of the background (22, 45, 47). Antibody titers were calculated on a log2 scale. Anti-LT and anti-STa antibody neutralization. The CT and STa toxin neutralizing activity of antibodies in rabbit serum and fecal samples was examined in T84 cells using cyclic AMP (cAMP) and cyclic GMP (cGMP) enzyme immunoassay kits (Assay Design, Ann Arbor, MI) as described previously (47). Briey, approximately 1 105 to 2 105 T84 cells (with greater than 80% conuence) were seeded into each well in Dulbeccos modied Eagle medium and Hams F12 medium (DMEM/F12; GIBCO/Invitrogen, Grand Island, NY). Ten nanograms of CT or 2 ng of STa toxin (diluted in 150 l of DMEM/F12) was incubated with 150 l of a rabbit serum or fecal sample (1:5 dilution in DMEM/F12, in triplicate) at room temperature. After 1 h of incubation, the mixture (150 l of the CT or STa toxin dilution plus 150 l of the serum or fecal sample dilution) was added to each well, and the plate was further incubated at 37C in 5% CO2 for 1 h. After a wash, the cells were lysed with 0.1 M HCl (200 l per well) and then neutralized with 0.1 M NaOH. Cell lysates were collected by centrifugation at 660 g for 10 min at room temperature. The resultant supernatant was tested for intracellular cAMP or cGMP levels by following the manufacturers protocols
1862
ZHANG AND ZHANG TABLE 2. E. coli strains and plasmids used in this study
Strain or plasmid Relevant property(ies)
CLIN. VACCINE IMMUNOL.
Source or reference
Strains TOP10 8851 8549 8616 8576 8069 3030-2 Plasmids pBAD88-102 p8069 pK88ac-LTB@EP3 pK88ac-LTB@EP4 pK88ac-STa@EP3 pK88ac-STa@EP3-LTB@EP4
F mcrA (mrr-hsdRMS-mcrBC) 80lacZ M15 lacX74 recA1 deoR araD139 (ara-leu)7697 galU galK rpsL (Strr) endA1 nupG TOP10/pK88ac-LTB@EP3 TOP10/pK88ac-LTB@EP4 TOP10/pK88ac-STa@EP3 TOP10/pK88ac-STa@EP3-LTB@EP4 TOP 10/p8069 (K88ac) Porcine ETEC isolate: K88ac LT STb K88ac operon in pBAD pBAD88-102 with SpeI site downstream of faeG gene K88ac with LTB epitope at EP3 of FaeG major subunit K88ac with LTB epitope at EP4 of FaeG major subunit K88ac with STa epitope at EP3 of FaeG major subunit K88ac with STa epitope at EP3 and LTB epitope at EP4 of FaeG major subunit
Invitrogen This This This This 44 12 3 44 This This This This study study study study
study study study study
(Assay Designs, Inc., Ann Arbor, MI). Rabbit anti-K88ac serum (a gift from Thomas Casey at the National Animal Disease Center, Ames, IA) was included as a control in this study. Anti-adhesin antibody adherence inhibition assay. Porcine small intestinal IPEC-J2 cells expressing K88ac receptors (21) and K88ac mbrial ETEC strain 3030-2 were used in adherence inhibition assays. IPEC-J2 cells (1 105) were seeded into each well of a 24-well tissue culture plate containing DMEM/F12 (GIBCO/Invitrogen). Strain 3030-2 bacteria cultured overnight on LB agar plates were harvested with swabs and suspended in PBS. The multiplicity-ofinfection ratio was set at 5 bacteria per IPEC-J2 cell. Prior to the assay, we estimated the quantity of IPEC-J2 cells in each well by dislodging the cells from a well with 100 l of 0.25% trypsin and counting them under a microscope. One hundred microliters of a strain 3030-2 bacterial cell suspension was mixed with 20- l rabbit serum or fecal samples and incubated at room temperature for 1 h on a shaker at 50 rpm. The mixture was added to IPEC-J2 cells and incubated at 37C in 5% CO2 for 1 h. IPEC-J2 cells were washed three times with PBS and dislodged by incubation with 0.25% trypsin (100 l per well) at 37C in 5% CO2 for 30 min. Dislodged cells were collected by centrifugation (15,000 g for 10 min) and resuspended in 1 ml PBS. The resuspension was serially diluted (103, 104, and 105), spread on LB agar plates, and cultured at 37C overnight. Colonies were counted, and adherence was calculated as the number of CFU/ml. Statistical analysis. Data were analyzed by using the mixed procedure (SAS for Windows, version 8; SAS Institute, Cary, NC) and Students t test. Different treatments were compared; the results were expressed as means standard deviations. Calculated P values of 0.05 were regarded as signicant when treatments were compared at a two-tailed distribution and with two-sample equal variance.
Proteins with a molecular mass of 27 kDa were detected in total protein extracts of strains 8551, 8549, 8576, and 8616 and of positive-control strain 8069 with anti-K88ac MAb hybridoma supernatant but not the negative-control TOP10 strain. When rabbit anti-CT antiserum was used, proteins of the same size (27 kDa) were detected in the 8551, 8549, and 8557 strains but not detected in strain 8616 or TOP10. Accordingly, chimeric proteins expressed by only the 8576 and 8616 strains were recognized by rabbit anti-STa antiserum. Chimeric K88ac mbriae were biosynthesized and assembled at the surface of each constructed strain. Expression of chimeric K88ac mbriae was veried by immunolabeling TEM with anti-K88ac MAbs and anti-CT and anti-STa antisera (Fig. 2). Anti-K88ac MAbs detected mbriae assembled at the 8551, 8549, 8576, and 8616 cell surface, but no gold particle-labeled mbriae were detected in the TOP10 control strain. Anti-CT antibodies detected assembled mbriae from the 8551, 8549, and 8576 strains, whereas anti-STa antibodies detected mbriae displayed at the 8576 and 8616 cell surface.
RESULTS Four strains were constructed for this study (Table 2). Strain 8551 expressed chimeric K88ac mbriae with the EP3 epitope at the FaeG major subunit replaced with the LTP1 epitope, and strain 8549 had EP4 replaced with the LTP1 epitope. Strain 8616 produced chimeric K88ac mbriae that had EP3 replaced with the STa13 toxoid epitope. Strain 8576 was the double mutant that had EP3 replaced with the STa13 toxoid epitope and EP4 replaced with the LTP1 epitope (Fig. 1B). Each constructed strain was veried for expression of the chimeric FaeG proteins and chimeric K88ac mbriae. Veried chimeric mbriae were harvested and used as antigens for rabbit immunization to determine anti-K88, anti-LT, and anti-STa immunogenicity. Chimeric FaeG proteins were expressed in each constructed strain. Chimeric FaeG major subunits were veried with antiK88ac MAbs and anti-CT and anti-STa antibodies (Fig. 1C).
FIG. 2. Immunolabeling TEM images showing biosynthesized K88ac chimeric mbriae of the 8551, 8549, 8576, and 8616 strains. All of the chimeric mbriae were recognized by hybridoma supernatant of anti-K88ac MAbs 36/41 and 30/17, whereas chimeric mbriae carrying the LTP1 and STa13 epitopes were detected by anti-CT and anti-STa antisera. Gold-conjugated (20-nm particle size) goat anti-mouse and anti-rabbit IgG antibodies were used as the secondary antibodies. A JEOL-1210 transmission electron microscope (magnication, 30,000) was used for examination. E. coli strain TOP10 was used as the negative control.
VOL. 17, 2010
K88ac-TOXIN FIMBRIAE INDUCE PROTECTIVE IMMUNITY
1863
FIG. 3. Antibody titration from serum and fecal samples from rabbits immunized with chimeric K88ac mbriae. (A) Rabbit serum anti-K88ac antibody titration. K88ac mbriae puried from ETEC wild-type strain 3030-2 were used as antigens to titrate anti-K88ac antibodies in an ELISA. (B) Anti-LT antibody measurement in GM1 ELISAs with puried CT (Sigma) as the antigen. (C) Anti-STa antibody titration from an STa ELISA with STa-ovalbumin conjugates as antigens. Rabbit serum and fecal samples in binary dilution were used as the primary antibodies in ELISAs, and HRP-conjugated goat anti-rabbit IgG and IgA (1:5,000) were used as the secondary antibodies. ODs of 0.4 (after subtraction of the background reading) were used to calculate antibody titers (in log2). Error bars indicate standard deviations. **, P 0.01; *, P 0.05.
Chimeric K88ac mbriae showed a signicant reduction in binding to K88ac receptor-positive brush borders. Although chimeric mbriae displayed at the cell surface were clearly visible under immunolabeled TEM, their K88ac receptor-positive brush border binding activity was signicantly reduced. The numbers of bacteria of strains 8551, 8549, 8576, and 8616 bound to each brush border were 3.1 1.4, 0.8 1.4, 0.7 1.1, and 4.4 2.1, respectively. The binding activity of these four constructed strains was signicantly lower than that of diarrheagenic ETEC strain 3030-2 (13.5 2.8; P 0.01). Chimeric K88ac mbriae elicited anti-LT, anti-STa, and anti-K88ac antibodies. Anti-K88ac, anti-LT, and/or anti-STa antibodies were detected in serum and fecal samples from rabbits immunized i.m. with chimeric K88ac mbriae (Fig. 3A to C). Anti-K88 IgG antibodies were detected at titers (in log2) of 8.46 0.08, 8.95 0.02, 7.66 0.06, and 10.04 0.07, respectively (Fig. 3A). Anti-K88ac secretory IgA (seIgA) antibodies were detected at titers of 6.23 0.08, 6.07 0.13, 6.03 0.04, and 6.23 0.04 in the feces of rabbits immunized with strain 8551, 8549, 8576, and 8616 chimeric mbriae. In contrast, no anti-K88ac antibodies were detected in the serum or fecal sample from the control rabbit (1.79 0.79 for IgG and 1.31 0.24 for seIgA). Anti-LT antibodies were developed in rabbits immunized with chimeric K88ac mbriae carrying the LTP1 epitope (Fig. 3B). Serum samples from rabbits immunized with strain 8551, 8549, and 8576 chimeric mbriae had anti-LT IgG antibodies detected at titers of 7.01 0.18, 7.78 0.10, and 6.9 0.02, whereas anti-LT IgA antibodies were detected at 4.1 0.27, 3.9 0.76, and 4.2 0.56, respectively. That was signicantly different from anti-LT IgG (1.5 0.4) or IgA (1.3 0.3) antibody detection in serum samples from the control rabbit (P 0.01). No anti-LT IgG (1.57 0.24) or IgA (1.6 0.34) antibodies were detected in serum samples from rabbits immunized with strain 8616 chimeric mbriae. Similarly, anti-LT seIgA antibodies were only detected in fecal samples from rabbits immunized with the chimeric mbriae of strains 8551 (5.1 0.18), 8549 (5.1 0.10), and 8576 (5.1 0.14). Anti-STa antibodies were detected in rabbits immunized
with chimeric mbriae expressing the STa13 toxoid epitope (Fig. 3C). Anti-STa IgG antibodies were detected in serum samples from rabbits immunized with strain 8576 (7.51 0.04) and 8616 (7.49 0.12) chimeric mbriae. Similarly, only serum samples from rabbits immunized with strain 8576 and 8616 chimeric mbriae had anti-STa IgA antibodies detected, at titers of 5.88 0.06 and 5.9 0.12, respectively. No anti-STa IgG (1.69 0.52, 1.54 0.40, 1.31 0.18) or anti-STa IgA (1.49 0.13, 1.51 0.38, 1.21 0.16) antibodies were detected in serum samples from rabbits immunized with strain 8551 and 8549 chimeric mbriae and the control rabbit. AntiSTa seIgA antibodies were detected in fecal samples from rabbits immunized with chimeric mbriae of strains 8576 (6.08 0.19) and 8616 (6.10 0.14). Anti-toxin antibodies in rabbit serum and fecal samples neutralized CT and STa toxins. CT (Sigma) was no longer able to stimulate an increase in the intracellular cAMP level in T84 cells after incubation with the serum or fecal samples from rabbits immunized with chimeric K88ac mbriae of the 8551, 8549, and 8576 strains. In contrast, the serum and fecal samples from rabbits immunized with strain 8616, the serum sample of the control rabbit, or culture medium did not prevent the same amount of CT (10 ng) from increasing cAMP levels in T84 cells (Fig. 4A). The cAMP concentrations in the T84 cells treated with CT that had been incubated with the serum of rabbits immunized with strain 8551, 8549, and 8576 mbriae were 0.52 0.01, 0.44 0.03, and 0.45 0.04 pmol/ml, respectively. The cAMP levels in the T84 cells incubated with CT that was pretreated with the fecal resuspension from rabbits immunized with strain 8551, 8549, and 8576 mbriae were 1.13 0.06, 1.89 0.13, and 2.12 0.13 pmol/ml. These cAMP levels were signicantly lower than those in T84 cells incubated with CT pretreated with serum samples from rabbits immunized with strain 8616 mbriae (10.85 0.20 pmol/me; P 0.01) and from the control rabbit (11.75 0.35 pmol/me; P 0.01) or CT treated with fecal samples from rabbits immunized with strain 8616 mbriae (10.6 0.75 pmol/me; P 0.01) and from the control rabbit (13.5 1.22 pmol/me; P 0.01).
1864
ZHANG AND ZHANG
CLIN. VACCINE IMMUNOL.
FIG. 4. Anti-LT and anti-STa antibody neutralization. Serum and fecal samples (1:5) from rabbits immunized with chimeric K88ac mbriae were used to neutralize 10 ng CT (A) or 2 ng STa (B). The mixture was added to T84 cells to detect stimulation of intracellular cGMP levels (pmol/ml) with a direct cGMP enzyme immunoassay kit (Assay Designs) to assess anti-STa antibody neutralizing activity and of intracellular cAMP levels with a direct cAMP enzyme immunoassay kit (Assay Designs) to measure anti-LT antibody neutralizing activity. Cell culture medium alone and serum samples from the control rabbit were also included. Error bars indicate standard deviations. **, P 0.01.
Similarly, antibodies in serum and fecal samples from rabbits immunized with K88ac-STa chimeric mbriae prevented STa toxin (2 ng) from stimulating cGMP levels in T84 cells (Fig. 4B). The intracellular cGMP concentration in T84 cells treated with STa and serum samples from rabbits immunized with strain 8576 and 8616 mbriae were 0.74 0.04 and 1.05 0.07 pmol/ml, respectively. These cGMP levels were signicantly lower than the cGMP levels in cells incubated with STa incubated with serum samples from rabbits immunized with mbriae from strain 8551 (54 2.83; P 0.01). Fecal samples from rabbits immunized with strain 8576 and 8616 mbriae also prevented STa toxin stimulation of cGMP levels, as T84 cells had cGMP levels of 4.2 0.42 and 5.4 0.57 pmol/ml, respectively. These cGMP levels were signicantly lower than the levels in T84 cells when fecal samples from rabbits immunized with strain 8551 mbriae (31 2.12 pmol/ml; P 0.01) or with culture medium alone (38 2.83 pmol/ml; P 0.01) were used to incubate STa toxin. To test whether anti-K88ac antibodies interfere with the stimulation of intracellular cAMP or cGMP in T84 cells by LT or STa, we used rabbit anti-K88ac serum samples to preincubate CT (10 ng) and STa (2 ng) in a separate study. Data from the cAMP ELISA showed similar cAMP levels (P 0.14) in T84 cells treated with LT alone (10 0.7 pmol/ml) and those treated with LT plus anti-K88ac serum (8.4 0.7 pmol/ml). Similarly, no signicant differences in cGMP stimulation were found when STa (26.8 2.5 pmol/ml) or STa plus rabbit anti-K88ac serum (24.3 2.5 pmol/ml) was used to incubate T84 cells (P 0.42). Antibodies inhibit the adherence of K88ac mbrial strain 3030-2 bacteria to K88ac receptors. Antibodies in serum and fecal samples from immunized rabbits reduced the adherence of K88ac mbrial ETEC strain 3030-2 bacteria to K88ac receptor-positive IPEC-J2 cells (Fig. 5). After incubation with serum samples from rabbits immunized with chimeric mbriae from the 8551, 8549, 8576, and 8616 strains, the numbers (103) of strain 3030-2 bacteria bound to IPEC-J2 cells were 101
21, 142 17, 157 17, and 210 14, respectively. These binding levels were signicantly lower than that in strain 3030-2 bacteria incubated with the serum sample from the control rabbit (512 18; P 0.01) or with cultural medium (550 17; P 0.01). After incubation with fecal samples from rabbits immunized with chimeric mbriae from the 8551 and
FIG. 5. Bacterial adherence inhibition by serum and fecal samples from rabbits immunized with chimeric K88ac mbriae. Porcine ETEC strain 3030-2 that expresses K88ac mbriae and porcine cell line IPEC-J2 expressing K88ac receptors were used in adherence inhibition assays. IPEC-J2 cells (1 105) were seeded into each well, and the multiplicity-of-infection ratio was preset at 5 bacteria per IPEC-J2 cell. After incubation with rabbit serum or fecal samples (1:5) at room temperature for 1 h, strain 3030-2 bacteria were added to each well containing IPEC-J2 cells and cultured at 37C in 5% CO2 for 1 h. Cells were dislodged, serially diluted in PBS, plated on LB agar plates, and incubated at 37C overnight. Colonies were counted and calculated as CFU/ml (in thousands). Error bars indicate standard deviations. **, P 0.01.
VOL. 17, 2010
K88ac-TOXIN FIMBRIAE INDUCE PROTECTIVE IMMUNITY
1865
8616 strains, strain 3030-2 showed a signicantly lower level of binding to IPEC-J2 cells (240 15 and 230 17, respectively; P 0.01). However, when incubated with fecal samples from rabbits immunized with mbriae from the 8549 and 8576 strains, strain 3030-2 bacteria showed only a slight reduction in binding to IPEC-J2 cells (470 18 and 460 15, respectively), which was not signicantly different from the binding to IPEC-J2 cells by strain 3030-2 bacteria treated with fecal sample of the control rabbit (490 12; P 0.38, P 0.21) or with the cultural medium (530 14; P 0.09, P 0.06). DISCUSSION ETEC adhesins that mediate bacterial attachment to host epithelial cells and LT and ST enterotoxins that induce uid and electrolyte hypersecretion in the host small intestine play key roles in ETEC-associated diarrhea. Therefore, control or prevention of ETEC infections has largely been based on two strategies: blocking bacterial adherence to host receptors and eliminating enterotoxin activity in host epithelial cells (29, 39). Experimental whole-cell vaccines derived from E. coli strains expressing an adhesin and a toxin antigen, or subunit vaccine candidates combining an adhesin antigen and a toxin antigen or carrying a adhesin-toxin fusion protein, could stimulate both anti-adhesin and anti-toxin immunity in hosts. Human volunteers, after administration of a mixture of puried E. coli surface antigen CS6 and LT toxoid LT192 proteins, developed anti-CS6 and anti-LT immunity (22). Similarly, after being immunized with a modied Shigella exneri 2 vaccine strain expressing ETEC CFA/I structural subunits and an LTB subunit, guinea pigs developed anti-CFA/I and anti-LT antibodies that showed the abilities to neutralize toxin and inhibit cell agglutination (30). Even when a native STa peptide was integrated into the ClpG major subunit of E. coli CS31A mbriae, neutralizing anti-STa antibodies and anti-ClpG antibodies were developed in immunized rabbits and mice (4, 5). Those studies indicated that anti-adhesin and anti-LT or anti-STa immunity can be induced in hosts by the coadministration of puried adhesin antigens and toxin antigens or administration of E. coli cells expressing adhesin and toxin antigens separately. Data from this study demonstrated that administration of chimeric adhesins that had LT and STa epitopes embedded induced not only anti-adhesin but also both anti-LT and antiSTa anti-toxin immunity. In this study, we genetically integrated the LTP1 epitope and the STa13 toxoid epitope into the FaeG major subunit of E. coli K88ac mbriae for the rst time to explore a mbrial platform vaccine that stimulates both anti-LT and anti-STa anti-toxin immunity and anti-adhesin immunity. As ETEC strains expressing either LT toxin or STa toxin can cause diarrhea (16, 28, 31, 35, 42, 46), inducing both anti-LT and anti-STa immunity is essential for effective protection against ETEC infection. By expressing both LT and STa epitopes at the same major subunit of K88ac mbriae, we produced a single antigen able to induce anti-K88ac mbrial immunity, anti-LT immunity, and anti-STa immunity. After being immunized with the chimeric mbriae from strain 8576 (K88ac-LTB epitope-STa13 epitope), rabbits developed antibodies that reduced K88ac mbrial adherence and neutralized CT and STa toxin stimulation of cAMP and cGMP in T84 cells. Knowing that induced
antibodies neutralizing toxins in vitro may not necessarily prevent delivery of toxin by living bacterial, we need to conduct animal challenge studies to assess induced anti-adhesin and anti-toxin immunity for in vivo protection against ETEC diarrhea. Also, further studies are needed to determine the minimum dose of induced antibodies required for toxin neutralization or adherence inhibition. Nevertheless, this study clearly demonstrated that anti-adhesin and anti-toxin (both anti-LT and anti-STa) immunity was induced and suggests the future application of a mbrial platform in ETEC vaccine development. The LTP1 epitope was selected in chimeric K88ac mbria construction because a homologous nontoxic CTP1 epitope elicited anti-CT antibodies when it was integrated into bacterial agella (10). Likewise, the selection of the STa13 epitope was based on the fact that the detoxied STa13 protein showed anti-STa immunogenicity when it was fused to a carrier protein, LT192 (47). Insertion of LTP1 either at EP3 (strain 8551) or at EP4 (strain 8549) of FaeG of K88ac apparently did not affect chimeric mbrial antigen elicitation of anti-LT antigenicity, as rabbits immunized with chimeric mbrial antigens from strains 8551 and 8549 developed nearly identical titers of anti-LT IgG and IgA antibodies. Furthermore, chimeric mbriae of the LTP1 single mutants (strain 8551 and 8549) and the LTP1/STa13 double mutant (strain 8576) elicited equivalent titers of anti-LT antibodies. Similarly, the same titers of anti-STa antibodies were elicited by chimeric mbriae expressing the STa13 epitope alone at EP3 (strain 8576) or with LTP1 at EP4 (strain 8616). These data indicate that the additional expression of the STa13 epitope at EP3 did not negatively affect the immunogenicity of the inserted LTP1 epitope, suggesting that this K88ac mbria could serve as a platform to express multiple foreign epitopes. E. coli strains expressing chimeric K88ac mbriae that had EP3 and EP4 of the FaeG subunit replaced with the LTP1 and STa13 epitopes can be used in future live attenuated vaccine development if expressed chimeric K88ac mbriae maintain host receptor binding activity. However, expression of multiple foreign epitopes could alter the K88ac mbrial structure or/ and biological activity, thus limiting its application in live vaccine strain development. Our previous study indicated that removal of EP3 or EP4 did not affect K88ac mbriae in binding to K88ac receptor-positive porcine brush borders (data not shown). It has been reported that the chimeric agella of Salmonella cells maintained mobility when CTP1 was expressed by the agella (10), and K88ab mbriae kept their binding function after their native epitopes were replaced with epitopes from the VP1 coat protein of the foot-and-mouth disease virus or a peptide from an envelope glycoprotein of human Immunodeciency virus type 1 (3, 27). After replacing EP3 and/or EP4 with LTP1 and/or STa13 peptides, we found, however, that the resultant chimeric K88ac mbriae showed a signicant reduction in binding activity compared to the native K88ac mbriae in strain 3030-2. A reduction in binding to K88ac receptors located on small intestinal epithelial cells likely could signicantly decrease the stimulation of host local mucosal immunity by these chimeric mbriae. Although their application in subunit vaccines, at least through the i.m. route, will not be affected, these K88ac-toxin chimeric mbriae may not be suitable for use as oral vaccine antigens. In addition,
1866
ZHANG AND ZHANG
CLIN. VACCINE IMMUNOL.
7. Berberov, E. M., Y. Zhou, D. H. Francis, M. A. Scott, S. D. Kachman, and R. A. Moxley. 2004. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect. Immun. 72:39143924. 8. Buccato, S., D. Maione, C. D. Rinaudo, G. Volpini, A. R. Taddei, R. Rosini, J. L. Telford, G. Grandi, and I. Margarit. 2006. Use of Lactococcus lactis expressing pili from group B Streptococcus as a broad-coverage vaccine against streptococcal disease. J. Infect. Dis. 194:331340. 9. Cattozzo, E. M., B. A. Stocker, A. Radaelli, C. De Giule Morghen, and M. Tognon. 1997. Expression and immunogenicity of V3 loop epitope of HIV-1, isolates SC and WMJ2, inserted in Salmonella agellin. J. Biotechnol. 56: 191203. 10. Chauhan, N., R. Kumar, J. Badhai, A. Preet, and P. K. Yasava. 2005. Immunogenicity of cholera toxin B epitope inserted in Salmonella agellin expressed on bacteria and administered as DNA vaccine. Mol. Cell. Biochem. 276:16. 11. Erickson, A. K., J. A. Willgohs, S. Y. McFarland, D. A. Beneld, and D. H. Francis. 1992. Identication of two porcine brush border glycoproteins that bind the K88ac adhesin of Escherichia coli and correlation of these glycoproteins with the adhesive phenotype. Infect. Immun. 60:983988. 12. Francis, D. H., and J. A. Willgohs. 1991. A live avirulent Escherichia coli vaccine for K88 enterotoxigenic colibacillosis in weaned pigs. Am. J. Vet. Res. 52:10511055. 13. Frantz, J. C., and M. W. Mellencamp. 1983. Production and testing of Escherichia coli (LTa) toxoid, p. 500517. In S. D. Acres (ed.), Proceedings of the Fourth International Symposium on Neonatal Diarrhea. VIDO Publications, Saskatoon, Saskatchewan, Canada. 14. Frantz, J. C., and D. C. Robertson. 1981. Immunological properties of Escherichia coli heat-stable enterotoxins: development of a radioimmunoassay specic for heat-stable enterotoxins with suckling mouse activity. Infect. Immun. 33:193198. 15. Gaastra, W., and A. M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 4:444452. 16. Girard, M. P., D. Steele, C. L. Chaignat, and M. P. Kieny. 2006. A review of vaccine research and development: human enteric infections. Vaccine 24: 27322750. 17. Hirayama, T. 1995. Heat-stable enterotoxin of Escherichia coli, p. 281296. In J. Moss, B. Iglewski, M. Vaughan, and A. T. Tu (ed.), Bacterial toxins and virulence factors in disease. Marcel Dekker, Inc., New York, NY. 18. Hol, W. G. J., T. K. Sixma, and E. A. Merritt. 1995. Structure and function of E. coli heat-labile enterotoxin and cholera toxin B pentamer, p. 185223. In J. Moss, B. Iglewski, M. Vaughan, and A. T. Tu (ed.), Bacterial toxins and virulence factors in disease. Marcel Dekker, Inc., New York, NY. 19. Hughes, J. M., F. Murad, B. Chang, and R. L. Guerrant. 1978. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature 271:755756. 20. Jobling, M. G., R. Rasulova, S. T. Poole, S. A. Sinocock, M. A. Davis, G. Price, S. J. Savarino, and R. K. Holmes. 2007. Optimized production and evaluation of pilus adhesin plus enterotoxin B subunit chimeras as novel candidate vaccines against diarrheal caused by enterotoxigenic E. coli, p. 203207. 42nd Annual U.S.-Japan Cholera and Other Bacterial Enteric Infections Joint Panel Meeting, Austin, TX. 21. Koh, S. Y., S. George, V. Brozel, R. Moxley, D. H. Francis, and R. S. Kaushik. 2008. Porcine intestinal epithelial cell lines as a new in vitro model for studying adherence and pathogenesis of enterotoxigenic Escherichia coli. Vet. Microbiol. 130:191197. 22. Lapa, J. A., S. A. Sincock, M. Ananthakrishnan, C. K. Porter, F. J. Cassels, C. Brinkley, E. R. Hall, J. Hamont, J. D. Gramling, C. M. Carpenter, S. Baqar, and D. R. Tribble. 2008. Randomized clinical trial assessing the safety and immunogenicity of oral microencapsulated enterotoxigenic Escherichia coli surface antigen 6 with or without heat-labile enterotoxin with mutation R192G. Clin. Vaccine Immunol. 15:12221228. 23. Moon, H. W. 1978. Mechanisms in the pathogenesis of diarrhea: a review. J. Am. Vet. Med. Assoc. 172:443448. 24. Moon, H. W. 1990. Colonization factor antigens of enterotoxigenic Escherichia coli in animals. Curr. Top. Microbiol. Immunol. 151:147165. 25. Moon, H. W., and T. O. Bunn. 1993. Vaccines for preventing enterotoxigenic Escherichia coli infections in farm animals. Vaccine 11:213220. 26. National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC. 27. Pedersen, P. A. 1991. Structure-function analysis of the K88ab mbrial subunit protein from porcine enterotoxigenic Escherichia coli. Mol. Microbiol. 5:10731080. 28. Qadri, F., S. K. Das, A. S. Faruque, G. J. Fuxhs, M. J. Albert, R. B. Sack, and A.-M. Svennerholm. 2000. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J. Clin. Microbiol. 38:2731. 29. Qadri, F., A. M. Svennerholm, A. S. Faruque, and R. B. Sack. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, and prevention. Clin. Microbiol. Rev. 18:465483. 30. Ranallo, R. T., C. R. Fonseka, F. Cassels, J. Srinivasan, and M. M. Ven-
epitope replacement could also inuence mbrial structure or stability, as it was shown in Fig. 1C that four chimeric mbriae had differences in interaction with anti-K88ac MAbs, although these differences could also be caused by the specicity of the MAbs used in this study. It has been noticed that anti-K88ac antibodies in serum samples from the immunized rabbits showed a similar inhibition of the adherence of strain 3030-2 bacteria to IPEC-J2 cells but antibodies in fecal samples from the immunized rabbits exhibited a signicant difference in adherence inhibition. Fecal samples from rabbits immunized with chimeric K88ac mbriae having EP3 replaced with the LTP1 or STa13 epitope showed signicant adherence inhibition, but antibodies in fecal samples from rabbits immunized with chimeric K88ac with EP4 substituted showed little adherence-inhibiting activity (Fig. 5). It is unclear what caused this variation in adherence inhibition, but we speculate that the replacement of EP4 likely altered the chimeric K88ac mbrial structure. Future studies to construct alternative chimeric K88ac mbriae by replacing EP4 with the STa13 toxoid peptide or a different LTB epitope and to evaluate the elicited antibodies in fecal samples in adherence inhibition will help us to construct a double mutant that elicits antibodies for better adherence inhibition. Expression of two or more toxin epitopes in K88ac mbriae could lead to the application of K88ac mbriae as a platform for developing effective multivalent vaccines against porcine ETEC diarrhea. All of the ETEC strains associated with porcine diarrhea express LT and STa or STb toxins, and nearly 70% of these strains express K88ac mbriae (48). In addition, K88ac mbriae, like other bacterial agella or mbriae, are excellent mucosal adjuvants and protein carriers (3, 4, 6, 810, 32). Thus, K88ac mbriae can be used as protein carriers to enhance the immunogenicity of otherwise non- or poorly immunogenic epitope antigens and to induce host mucosal immunity. K88ac mbriae, like other bacterial adhesins, may also serve as platforms for developing epitope vaccines against other enteric diseases.
ACKNOWLEDGMENTS We thank Eric Nelson and Craig Welbon for their assistance in rabbit immunization, David Francis for providing anti-K88ac MAb hybridoma supernatant, Don Robertson for STa conjugates and antiSTa antiserum, and Robert Japas for assistance with TEM. Financial support for this study was provided by NIH AI068766 (W. Zhang), the Center for Infectious Disease Research and Vaccinology, and the South Dakota Agricultural Experiment Station.
REFERENCES 1. Alexander, T. J. L. 1994. Neonatal diarrhoea in pigs, p. 151170. In C. L. Gyles (ed.), Escherichia coli in domestic animals and human. CAB International, Oxon, United Kingdom. 2. Ausubel, F. M., R. Brent, R. K. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1999. Short protocols in molecular biology, 4th ed. John Wiley & Sons, Inc., New York, NY. 3. Bakker, D., van F. G. Zijderveld, S. van der Veen, B. Oudega, and F. K. de Graaf. 1990. K88 mbriae as carriers of heterologous antigenic determinants. Microb. Pathog. 8:343352. 4. Batisson, I., and M. Der Vartinian. 2000. Contribution of dened amino acid residues to the immunogenicity of recombinant Escherichia coli heat-stable enterotoxin fusion proteins. FEMS Microbiol. Lett. 192:223229. 5. Batisson, I., M. Der Vartanian, B. Gaillard-Martinie, and M. Contrepois. 2000. Full capacity of recombinant Escherichia coli heat-stable enterotoxin fusion proteins for extracellular secretion, antigenicity, disulde bond formation, and activity. Infect. Immun. 68:40644074. 6. Ben-Yedidia, T., and R. Arnon. 2006. Flagella as a platform for epitopebased vaccines. Isr. Med. Assoc. J. 8:316318.
VOL. 17, 2010
K88ac-TOXIN FIMBRIAE INDUCE PROTECTIVE IMMUNITY
1867
31.
32.
33. 34.
35.
36.
37.
38.
39.
katesan. 2005. Construction and characterization of bivalent Shigella exneri 2 vaccine strains SC608(pCFAI) and SC608(pCFAI/LTB) that express antigens from enterotoxigenic Escherichia coli. Infect. Immun. 73:258267. Reischl, U., M. T. Youssef, H. Wolf, E. Hyytia-Trees, and N. A. Strockbine. 2004. Real-time uorescence PCR assays for detection and characterization of heat-labile I and heat-stable I enterotoxin genes from enterotoxigenic Escherichia coli. J. Clin. Microbiol. 42:40924100. Sbrogio-Almeida, M. E., T. Mosca, L. M. Massis, I. A. Abrahamsohn, and L. C. Ferreira. 2004. Host and bacterial factors affecting induction of immune responses to agellin expressed by attenuated Salmonella vaccine strains. Infect. Immun. 72:25462555. Sears, C. L., and J. B. Kaper. 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol. Rev. 60:167215. Sellwood, R., R. A. Gibbons, G. W. Jones, and J. M. Rutter. 1975. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J. Med. Microbiol. 8:405411. Shaheen, H. I., S. B. Khalil, M. R. Rao, R. A. Elyazeed, T. F. Wierzba, L. F. Peruski, Jr., S. Putnam, A. Navarro, B. Z. Morsy, A. Cravioto, J. D. Clemens, A.-M. Svennerholm, and S. J. Savarino. 2004. Phenotypic proles of enterotoxigenic Escherichia coli associated with early childhood diarrhea in rural Egypt. J. Clin. Microbiol. 42:55885595. Sizemore, R. R., K. L. Roland, and U. S. Ryan. 2004. Enterotoxigenic Escherichia coli virulence factors and vaccine approaches. Expert Rev. Vaccines 3:585595. Smith, H. W., and M. A. Linggood. 1971. Observation on the pathogenic properties of the K88, HIY and ENT plasmids of Escherichia coli with particular reference to porcine diarrhea. J. Med. Microbiol. 4:467485. Sun, R., T. J. Anderson, A. K. Erickson, E. A. Nelson, and D. H. Francis. 2000. Inhibition of adhesion of Escherichia coli K88ac mbria to its receptor, intestinal mucin-type glycoproteins, by a monoclonal antibody directed against a variable domain of the mbria. Infect. Immun. 68:35093515. Svennerholm, A. M., and D. Steele. 2004. Microbial-gut interactions in health and disease. Progress in enteric vaccine development. Best Pract. Res. Clin. Gastroenterol. 18:421445.
40. Van den Broeck, W., E. Cox, and B. M. Goddeeris. 1999. Induction of immune responses in pigs following oral administration of puried F4 mbriae. Vaccine 17:20202029. 41. Wilson, M. R., and D. H. Francis. 1986. Fimbriae and enterotoxins associated with Escherichia coli serogroups isolated from pigs with colibacillosis. Am. J. Vet. Res. 47:213217. 42. Wolf, M. K. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxin of enterotoxigenic Escherichia coli. Clin. Microbiol. Rev. 10:569584. 43. Zhang, W., E. M. Berberov, J. Freeling, D. He, R. A. Moxley, and D. H. Francis. 2006. Signicance of heat-stable and heat-labile enterotoxins in porcine colibacillosis in an additive model for pathogenicity studies. Infect. Immun. 74:31073114. 44. Zhang, W., Y. Fang, and D. H. Francis. 2009. Characterization of the binding specicity of K88ac and K88ad mbriae of enterotoxigenic Escherichia coli by constructing K88ac/K88ad chimeric FaeG major subunits. Infect. Immun. 77:699706. 45. Zhang, W., and D. H. Francis. 2010. Genetic fusions of heat-labile toxoid (LT) and heat-stable toxin b (STb) of porcine enterotoxigenic Escherichia coli elicit protective anti-LT and anti-STb antibodies. Clin. Vaccine Immunol. 17:12231231. 46. Zhang, W., D. C. Robertson, C. Zhang, W. Bai, M. Zhao, and D. H. Francis. 2008. Escherichia coli constructs expressing human or porcine enterotoxins induce identical diarrheal diseases in a piglet infection model. Appl. Environ. Microbiol. 74:58325837. 47. Zhang, W., C. Zhang, D. H. Francis, J. Nataro, and D. C. Robertson. 2010. Genetic fusions of pLT192 and pSTa toxoids of enterotoxigenic Escherichia coli (ETEC) induce neutralizing anti-LT and anti-STa antibodies. Infect. Immun. 78:316325. 48. Zhang, W., M. Zhao, L. Ruesch, A. Omot, and D. H. Francis. 2007. Prevalence of virulence genes in Escherichia coli strains isolated from young pigs with diarrhea in the U.S. Vet. Microbiol. 123:145152.
You might also like
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- 2022 2024 Syllabus PDFDocument16 pages2022 2024 Syllabus PDFViditi DhaddaNo ratings yet
- Genetic Control of ProteinDocument43 pagesGenetic Control of ProteinGabriela ZahiuNo ratings yet
- Role of The Cytoskeletal Proteins RMD On Chloroplast Response To LightDocument9 pagesRole of The Cytoskeletal Proteins RMD On Chloroplast Response To Light18217426462No ratings yet
- Student's Copy Chapter3 BioDocument10 pagesStudent's Copy Chapter3 BioSaidatul Atyah Mohd ApendaiNo ratings yet
- Lecture 01 Notes 5.07Document7 pagesLecture 01 Notes 5.07akiridoNo ratings yet
- Four Major Drug TargetsDocument34 pagesFour Major Drug TargetsFlowerNo ratings yet
- 1 Unit1Document8 pages1 Unit1Rajeev SinghNo ratings yet
- DLL Do SCI g10 Q3 Week6 22 23Document8 pagesDLL Do SCI g10 Q3 Week6 22 23NURSHAHADAH ISMAELNo ratings yet
- GLIKOGENESISDocument45 pagesGLIKOGENESISChrista24796No ratings yet
- Northern & Southern BlotsDocument17 pagesNorthern & Southern BlotsShawon RahmanNo ratings yet
- MCQ 2 - PharmacodynamicsDocument5 pagesMCQ 2 - PharmacodynamicsHillary Nguyen100% (3)
- Engineering Metabolism Through Dynamic Control: SciencedirectDocument11 pagesEngineering Metabolism Through Dynamic Control: SciencedirectShampa SenNo ratings yet
- 323 FullDocument6 pages323 FulljosekinNo ratings yet
- Earth and Life Science NotesDocument3 pagesEarth and Life Science Notesradz quilinganNo ratings yet
- Structural Analysis of Sequence Data in Virology An Elementary Approach Using Sars-Cov-2 As An ExampleDocument35 pagesStructural Analysis of Sequence Data in Virology An Elementary Approach Using Sars-Cov-2 As An ExampleAdrian BoboceaNo ratings yet
- First Aid For The Basic Sciences General Principles 3Rd Edition Tao Le Full ChapterDocument67 pagesFirst Aid For The Basic Sciences General Principles 3Rd Edition Tao Le Full Chapterfelix.atkinson425100% (16)
- Significance and Importance of Biochemistry in Nursing: UNIT-1Document7 pagesSignificance and Importance of Biochemistry in Nursing: UNIT-1Sunil PatelNo ratings yet
- G11 Biology chap1 3 - 知识点整理和默写卷Document21 pagesG11 Biology chap1 3 - 知识点整理和默写卷晁海瀚No ratings yet
- Electron Transfer in BiologyDocument20 pagesElectron Transfer in BiologyVani KaushikNo ratings yet
- Sparvix Publishing House ProjectDocument100 pagesSparvix Publishing House ProjectDhiraj PatilNo ratings yet
- Bioenergetics and Oxidative PhosphorylationDocument32 pagesBioenergetics and Oxidative PhosphorylationShimmering MoonNo ratings yet
- 432515250219pertemuan Vi Dan Vii Introduction of Dna Into Living Cell-1Document21 pages432515250219pertemuan Vi Dan Vii Introduction of Dna Into Living Cell-1Sella Amelia PuteriNo ratings yet
- BM 1 1 CytoskeletonDocument24 pagesBM 1 1 CytoskeletonSanthoshi Sadhanaa SankarNo ratings yet
- HLA & HLA Antigen Typing MethodsDocument53 pagesHLA & HLA Antigen Typing Methodsbetsy jonahsNo ratings yet
- Glioblastoma Five Cases Positive For Borellia FISH Probes Alan MacDonald DarvishDocument17 pagesGlioblastoma Five Cases Positive For Borellia FISH Probes Alan MacDonald DarvishteslatikaNo ratings yet
- Biology Word SearchDocument1 pageBiology Word SearchreynandcpcNo ratings yet
- Quiz Lipids and LipoproteinsDocument9 pagesQuiz Lipids and LipoproteinsAllyah Ross DuqueNo ratings yet
- DNA MutationsDocument44 pagesDNA MutationsYashika A.No ratings yet
- Practical 4 Protein SpectrophotometryDocument5 pagesPractical 4 Protein SpectrophotometryRhulani MakhubelaNo ratings yet
- RAPD-PCR Based Marker Approach For The Genetic Differentiation of Two Species of Cockroach (Order-Dictyoptera)Document6 pagesRAPD-PCR Based Marker Approach For The Genetic Differentiation of Two Species of Cockroach (Order-Dictyoptera)LifedavidNo ratings yet