Professional Documents
Culture Documents
Titanium
Uploaded by
potterhead1DOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Titanium
Uploaded by
potterhead1DCopyright:
Available Formats
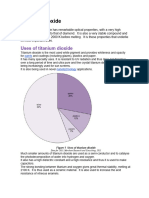
Titanium Uses
Due to excellent resistance to sea water, it is used to make propeller shafts and rigging and in the heat exchangers of desalination plants and in heater-chillers for salt water aquariums. Owing to its high stiffness it is favoured in place of steel in high performance model sailplane wing join rods. It is used to produce relatively soft artificial gemstones. Titanium tetrachloride is used as an iridized coating for glass. Because it fumes strongly in moist air it is also used for skywriting! Titanium is an important pigment for industrial, domestic and artistic applications, as it is extremely opaque and sun fast. This latter property is also exploited in sunscreen applications. Because it is physiologically inert and can Osseo integrate, titanium is a choice material for joint replacement and tooth implants, and body piercing. Titanium alloys are also used in eyeglass frames. This results high-end, but resistant and longlasting frame. Both traditional alloys and shape memory alloys find use in this application. Even golf clubs are made with titanium. Even architectural applications of titanium exist, for instance the Guggenheim and the Cerritos Library that are sheathed in titanium panels. Apple computers recently put a titanium laptop computer on the market, for its light weight. Titanium has potential use in desalination plants for converting sea water into fresh water. How is Titanium extracted? You cant use carbon reduction so that means that you have to use an alternative reducing agent. In the case of titanium, the reducing agent is either sodium or magnesium. Both of these would, of course, first have to be extracted from their ores by expensive processes.
Conversion of titanium(IV) oxide, TiO2, into titanium(IV) chloride, TiCl4 The ore retile (impure titanium(IV) oxide) is heated with chlorine and coke at a temperature of about 900C.
Other metal chlorides are formed as well because of other metal compounds in the ore. Very pure liquid titanium(IV) chloride can be separated from the other chlorides by fractional distillation under an argon or nitrogen atmosphere, and is stored in totally dry tanks.
Note: Titanium(IV) chloride is a typical covalent chloride. It is a colourless liquid which fumes in moist air due to reaction with water to give titanium(IV) oxide and fumes of hydrogen chloride. Everything has to be kept very dry to prevent this happening.
Reduction of the titanium(IV) chloride Reduction by sodium This is the method which is used in the UK. The titanium(IV) chloride is added to a reactor in which very pure sodium has been heated to about 550C - everything being under an inert argon atmosphere. During the reaction, the temperature increases to about 1000C.
After the reaction is complete, and everything has cooled (several days in total - an obvious inefficiency of the batch process), the mixture is crushed and washed with dilute hydrochloric acid to remove the sodium chloride.
Note: To save you the bother of asking, I have no idea why hydrochloric acid is used to do this rather than just water!
Reduction by magnesium This is the method used in the rest of the world. The method is similar to using sodium, but this time the reaction is:
The magnesium chloride is removed from the titanium by distillation under very low pressure at a high temperature.
You might also like
- New Microsoft PowerPoint PresentationDocument31 pagesNew Microsoft PowerPoint PresentationASHRAFUL KABIRNo ratings yet
- Kroll ProcessDocument5 pagesKroll ProcessNavarro SalgadoNo ratings yet
- Powerpoint Presentation On Extraction of TitaniumDocument31 pagesPowerpoint Presentation On Extraction of TitaniumLuis Sahoo90% (10)
- Uses and Properties of Titanium DioxideDocument5 pagesUses and Properties of Titanium DioxideAbdul RazzaqueNo ratings yet
- Titanium T1Document2 pagesTitanium T1ThunderkickNo ratings yet
- Manufacture of Titanium DioxideDocument52 pagesManufacture of Titanium DioxideJayraj Dayma100% (1)
- Review Extractive Metallurgy Titanium - Daffa HandyanDocument6 pagesReview Extractive Metallurgy Titanium - Daffa HandyanDaffa HandyanNo ratings yet
- TiO2 Titanium Dioxide Extraction Production Project PresentationDocument48 pagesTiO2 Titanium Dioxide Extraction Production Project Presentationkaranved780% (5)
- Piru Seminar ReportDocument52 pagesPiru Seminar Reportsujit_sekharNo ratings yet
- Aerospace Materials Assignment 1 Titanium Metal and It's Application in Aerospace IndustryDocument7 pagesAerospace Materials Assignment 1 Titanium Metal and It's Application in Aerospace IndustryBilal MalikNo ratings yet
- TITANIUM DIOXIDE Chemical and Technical AssessmentDocument8 pagesTITANIUM DIOXIDE Chemical and Technical AssessmentDi Stovall100% (1)
- Titanium Corrosion PDFDocument3 pagesTitanium Corrosion PDFSellappan MuthusamyNo ratings yet
- HAZMAT Articles - Titanium TetrachlorideDocument2 pagesHAZMAT Articles - Titanium TetrachlorideRadko BankrasNo ratings yet
- Everything You Need to Know About TitaniumDocument20 pagesEverything You Need to Know About TitaniumAriska AndrainiNo ratings yet
- Titanium DioxideDocument18 pagesTitanium DioxidePrrinisha KanabathyNo ratings yet
- Pereperation of TitaniumDocument2 pagesPereperation of TitaniumTanish100% (1)
- Titaniun Dioxide Basic InformationDocument4 pagesTitaniun Dioxide Basic Informationalexandria.padilla11No ratings yet
- Anor TitaniumDocument16 pagesAnor Titaniumcahya larasatiNo ratings yet
- 16-04-2020-1587032337-6 - Ijce-2. Ijce - Processing of Rutile Concentrates by FluorinationDocument18 pages16-04-2020-1587032337-6 - Ijce-2. Ijce - Processing of Rutile Concentrates by Fluorinationiaset123No ratings yet
- Applications of Titanium TitaniumDocument1 pageApplications of Titanium TitaniumRicardo Rovira ChalerNo ratings yet
- History of TitaniumDocument10 pagesHistory of TitaniumsanmiteNo ratings yet
- Titanium Alloys and Its PropertiesDocument26 pagesTitanium Alloys and Its PropertiesAnand Prabhu100% (1)
- History of TitaniumDocument12 pagesHistory of TitaniumjagadeesanbNo ratings yet
- Recent Progress in Titanium Extraction and RecyclingDocument14 pagesRecent Progress in Titanium Extraction and Recyclingraihan dzakyNo ratings yet
- Production of the white pigment titanium dioxideDocument16 pagesProduction of the white pigment titanium dioxidehaisamdo100% (1)
- Titanium 1Document12 pagesTitanium 1Farid AhmadiNo ratings yet
- Highly Pure Thorium Nitrate from SulfateDocument4 pagesHighly Pure Thorium Nitrate from SulfateGyan PrameswaraNo ratings yet
- Life Cycle Assessment Comparison of Emerging and Traditional Titanium Dioxide Manufacturing ProcessesDocument11 pagesLife Cycle Assessment Comparison of Emerging and Traditional Titanium Dioxide Manufacturing ProcessesFelipe TavaresNo ratings yet
- Hydrometallurgy: Scott Middlemas, Z. Zak Fang, Peng FanDocument7 pagesHydrometallurgy: Scott Middlemas, Z. Zak Fang, Peng FanmonisalesNo ratings yet
- Mini Talk ChemistryDocument11 pagesMini Talk ChemistryMaria Alison SwiftNo ratings yet
- Cutting Tools Materials PDFDocument27 pagesCutting Tools Materials PDFAhmad DanielNo ratings yet
- Titanium Molten Salt ElectrolysisDocument14 pagesTitanium Molten Salt ElectrolysismtanaydinNo ratings yet
- Lecture 4 - Titanium GroupDocument31 pagesLecture 4 - Titanium Groupmalenya1No ratings yet
- Schm312 NotesDocument97 pagesSchm312 NotesSandile SynthaxError Mabika100% (1)
- Titanium Chloride As A CatalystDocument1 pageTitanium Chloride As A CatalysthadassahhadidNo ratings yet
- Technologies On SuperTitaniumDocument5 pagesTechnologies On SuperTitaniummeor3705No ratings yet
- Environmental Problems of Finely Dispersed Titanium Dioxide ProductionDocument13 pagesEnvironmental Problems of Finely Dispersed Titanium Dioxide ProductionDharamNo ratings yet
- ChemistryDocument20 pagesChemistryanisa aliNo ratings yet
- Paper Cloracion y Reduccion Con MagnesioDocument3 pagesPaper Cloracion y Reduccion Con MagnesioBryan Roncal LlajarunaNo ratings yet
- Titanium: A Strong yet Lightweight MetalDocument16 pagesTitanium: A Strong yet Lightweight Metalrifaldy100% (1)
- Titanio y AleacionesDocument7 pagesTitanio y AleacionesMarioNo ratings yet
- Sulfate Digestion Process For High Purity Tio From Titania SlagDocument6 pagesSulfate Digestion Process For High Purity Tio From Titania SlagQuang VANo ratings yet
- Titanium : Karan Saxena Class Xi-A ROLL NO.-22Document18 pagesTitanium : Karan Saxena Class Xi-A ROLL NO.-22Karan SaxenaNo ratings yet
- Oxide Titanium Ti O PigmentDocument10 pagesOxide Titanium Ti O Pigmentapi-27149699No ratings yet
- Material of The SR71Document6 pagesMaterial of The SR71zewdie.nataniaNo ratings yet
- A Literature Review of Titanium Metallurgical ProcessesDocument12 pagesA Literature Review of Titanium Metallurgical ProcessesMargarita CaceresNo ratings yet
- Benelite Process For Upgradation of IllemniteDocument6 pagesBenelite Process For Upgradation of Illemnitemadangk100% (1)
- Production of titanium tetrachloride (TiCl4) from titanium ores: A reviewDocument49 pagesProduction of titanium tetrachloride (TiCl4) from titanium ores: A reviewrazor75apNo ratings yet
- Application Notes Titanium EnglishDocument6 pagesApplication Notes Titanium EnglishXEFTAXNo ratings yet
- Extracting Copper from OresDocument25 pagesExtracting Copper from OresBalachandran P KamathNo ratings yet
- Niobium Processing NotesDocument5 pagesNiobium Processing NotesMarto KarezNo ratings yet
- Titanium The ChoiceDocument24 pagesTitanium The Choiceinfo7595100% (1)
- Aluminium and Titanium PowerpointDocument9 pagesAluminium and Titanium PowerpointKaizerani FatinNo ratings yet
- Processes For Recycling: 4.4.1.2.1 Conventional Kroll ProcessDocument14 pagesProcesses For Recycling: 4.4.1.2.1 Conventional Kroll Processelma watNo ratings yet
- SSRN Id4553127Document5 pagesSSRN Id4553127ghinasaleh97No ratings yet
- Kang 2016Document8 pagesKang 2016mylover huNo ratings yet
- 10.1016 J.jallCOM.2005.10.048 Extraction of Titanium From Different Titania Precursors by The FFC Cambridge ProcessDocument9 pages10.1016 J.jallCOM.2005.10.048 Extraction of Titanium From Different Titania Precursors by The FFC Cambridge ProcessHooman BaghbanNo ratings yet
- Nano Titania PowerPoint Presentationد رضويDocument40 pagesNano Titania PowerPoint Presentationد رضويArilu2010No ratings yet
- Elminster's Ecology Appendix 2Document74 pagesElminster's Ecology Appendix 2stefano vitelloneNo ratings yet
- 1Document168 pages1Gerry Dan ChanliongcoNo ratings yet
- Ultrasonic Algae ControlDocument12 pagesUltrasonic Algae ControlLisa brandNo ratings yet
- US20120103906 Closed Circuit Desalination Retrofit For Improved Performance of Common Reverse Osmosis SystemsDocument11 pagesUS20120103906 Closed Circuit Desalination Retrofit For Improved Performance of Common Reverse Osmosis SystemsWendyNo ratings yet
- Environmental Law Project WorkDocument11 pagesEnvironmental Law Project Workbinny kumariNo ratings yet
- Econocast 30 SdsDocument4 pagesEconocast 30 SdsJosue MorenoNo ratings yet
- Oil Production in OmanDocument17 pagesOil Production in OmanFarah Syeda0% (1)
- 01 Ind Iee 04Document221 pages01 Ind Iee 04Tony JosephNo ratings yet
- MSDS N-Butanol ArkemaDocument6 pagesMSDS N-Butanol Arkemaherry prasetyoNo ratings yet
- Hydrographs USDA SCSDocument50 pagesHydrographs USDA SCSdiebuisaNo ratings yet
- Borewell Survey Report - AndamanDocument40 pagesBorewell Survey Report - AndamandennysonNo ratings yet
- Cigarette Butts A Future Voltaic CellDocument9 pagesCigarette Butts A Future Voltaic CellTelle TelleNo ratings yet
- General Motors LawsuitDocument27 pagesGeneral Motors LawsuitAnonymous 5DZE7gzUnNo ratings yet
- A History of Early Comfort Cooling Using IceDocument7 pagesA History of Early Comfort Cooling Using IceLuis AlonsoNo ratings yet
- Types of Construction WorkDocument6 pagesTypes of Construction WorkKalsoma CarlsNo ratings yet
- 1 - API 571 (19 DMS)Document42 pages1 - API 571 (19 DMS)Mohammed Kadhim100% (2)
- Separating Mixtures: Sedimentation and DecantationDocument16 pagesSeparating Mixtures: Sedimentation and DecantationChloeNo ratings yet
- Daniel Alemayehu PDFDocument96 pagesDaniel Alemayehu PDFBeza AbebeNo ratings yet
- Soil ConservationDocument12 pagesSoil ConservationNicole ParaisoNo ratings yet
- SDS Mowilex Woodstain - EngDocument5 pagesSDS Mowilex Woodstain - EngMat ZubairNo ratings yet
- Chemical Waste Management GuidelinesDocument5 pagesChemical Waste Management GuidelinesSolomon SundayNo ratings yet
- Venturi Scrubber With Mist EliminatorDocument13 pagesVenturi Scrubber With Mist Eliminatorandy123hi100% (2)
- Aeration Types of AeratorsDocument2 pagesAeration Types of AeratorsMichael Asmith UniqueNo ratings yet
- English Presentation .Document16 pagesEnglish Presentation .Joshua Tazeem100% (1)
- Waste Water Treatment TechDocument23 pagesWaste Water Treatment TechArchit Gupta100% (1)
- Internship Report HarangiDocument104 pagesInternship Report HarangiHarsha DharmapalNo ratings yet
- Ac 150 5220-18a PDFDocument22 pagesAc 150 5220-18a PDFsahanNo ratings yet
- Example 2Document4 pagesExample 2ashu100% (1)
- 03 Int Brazil Stages1to6 ScienceDocument21 pages03 Int Brazil Stages1to6 ScienceThiago Henrique SantosNo ratings yet
- Presentation On Industrial Water Circulation SystemDocument19 pagesPresentation On Industrial Water Circulation Systembashok87No ratings yet