Professional Documents
Culture Documents
Anti-Anemia Drugs
Uploaded by
Adrul FauzanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Anti-Anemia Drugs
Uploaded by
Adrul FauzanCopyright:
Available Formats
Anti-Anemia Drugs
Anemia
2nd most presenting manifestation of disease, with pain being the first. It is defined as: low hemoglobin, low RBC count and low RBC mass. Usually presents with pallor, fatigability, weakness and pale conjunctivae In order to properly treat the anemia, you must determine the cause.
Causes of Anemia
1. Diminished production and or replacement of red blood cells.
2. Excessive breakdown and loss of red blood cells. Hemodilution while not a cause of anemia, it does cause an anemia-like effect.
1. Diminished Production/Replacement of RBCs Anemia's
Microcytic anemia deficiency of Fe
RBCs appear pale and smaller, and we see more reticulocytes in circulation. Can be caused by the chronic use of aspirin, which irritates the stomach GI blood loss.
Normocytic anemia deficiency of Erythropoietin
Caused by compromised renal function.
Macrocytic Anemia- deficiency of folic acid and B12
Diminished cell division and release of larger cells in circulation.
2. Breakdown of RBCs Anemia
Bleeding: can be due to an ulcer or in females blood loss due to their menstrual cycle Use of drugs that irritate the GI tract (aspirin) Hemolysis (Hemolytic Anemia) can be caused by:
Autoimmune disease Mechanical (heart valves, microvascular disease) Toxins (e.g., snake venom)
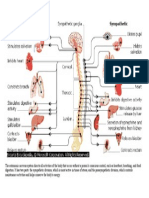
Sites of action for EPO
Therapeutic Uses of EPO
Anemia of end stage renal disease To treat AIDS anemia caused by AZTs suppression of bone marrow Anemia related to cancer chemotherapy Others
To increase RBC levels for autologous blood donation Anemia associated with rheumatoid arthritis
Biological Actions of Other Hematopoietic Growth Factors
1. Granulocyte/Macrophage Colony Stimulating Factor (GM-CSF)- Sargramostim
Acts synergistically with IL-3 to stimulate the formation and proliferation of colony forming cells: CFU-GEMM, BFU-E, CFU-Meg, CFU-GM, CFU-M, CFU-E Increases cytotoxic phagocytic activity of mature granulocytes
2. Interleukin 3 (IL-3)
Acts synergistically with GM-CSF to stimulate the formation of granulocytes, macrophages, eosinophils and megakaryocytes. Acts synergistically with EPO to stimulate formation of BFU-E colonies Induces CFU-S and leukemic blast cells into cell cycle
More Hematopoietic Growth Factors
3. Colony stimulating Factor-1 (CSF-1 or M-CSF)
Acts synergistically with GM-CSF and IL-3 to stimulate monocyte/macrophage colony formation and function
4. Granulocyte Colony Stimulating Factor (G-CSF) - filgrastim
Acts synergistically with IL-3, GM-CSF and CSF-1 to
stimulate formation of megakaryocytes, granulocytemacrophage and high proliferative potential (HPP) colonies Induces release of granulocytes from marrow
More Hematopoietic Growth Factors
5. Thrombopoietin (TSF)
Increases the size and number of megakaryocytes. (IL-11 also useful in stimulating production)
Increases the concentration of early megakaryocytes cells (SACHE+cells) in bone marrow. Produces an increase in megakaryocytes endomitosis.
Increases platelet size and number in plasma.
Iron Cycle
5 - 10% of ingested iron is absorbed
Once ingested the acid in the stomach:
1. Aids in ionization of iron 2. Splits chelated food iron from chelator 3. Maintains iron in soluble form 4. Allows iron to remain in the absorbable form Fe3+
Mechanism of Iron Absorption
Therapeutic uses of Iron
Iron Deficient Anemia Pregnancy Premature Babies
Hookworn infestation Malabsorption Syndrome GI Bleeding due to:
Ulcers Aspirin Excess consumption of coffee
Blood loss
Iron Preparations
Oral Iron
Ferrous Sulfate (Feosol) 300 mg tid Side Effects are extremely mild:
Nausea, upper abdominal pain, constipation or diarrhea.
Cheapest form of Iron and one of the most widely used
Parenteral
Iron Dextran (Imferon) IM or IV Indicated for patients who cannot tolerate or absorb oral iron or where oral iron is insufficient to treat the condition ie. Malabsorption syndrome, prolonged salicylate therapy, dialysis patients
Toxicity of Iron Overdose
5000 deaths/year in the US, usually in children 20% of children presenting with iron toxicity will die 1 to 2 grams are sufficient to cause death At high doses, Iron is absorbed through passive diffusion with no regulation
Iron Clinical Effects
Early changes
Vomiting, diarrhea Blood Volume HR TPR (reflex) Acidosis from Iron oxidation, Krebs cycle and anaerobic metabolism citric acid and lactic acid
Intermediate changes
Improvement (short lived) profound shock and CV Collapse Hepatic Failure, jaundice, pulmonary edema and death
Late Stage
Intestinal scarring, fatty acid degeneration of liver, cirrhosis and death.
Treatment of Iron Overdose
Toxic levels
ALD 200-300mgkg, plasma iron > 300ug/dl
ABCs supportive care Bicarbonate for acidosis Fluids for blood loss Ipecac or lavage
Chelation with Deferoxamine
Vitamin B12
Source: In food, especially in liver and kidneys. GI Microorganism synthesis, Vitamin Supplements (Cyanocobalamin) Necessary for normal DNA synthesis Absorption of B12
1. Intrinsic Factor (low dose): a protein made by stomach parietal cells that binds to B12 and delivers it from the ileum via a calcium mediated event. 2. Mass Action (High dose): 1000mg/day, absorbed via passive diffusion
B12 Deficiency
A B12 deficiency will cause peripheral neuropathy and a macrocytic anemia, a pernicious anemia. Folic Acid administration can correct the macrocytic anemia but will fail to correct the peripheral neuropathy.
To treat the neuropathy, Vit B12 must be utilized.
Mechanism for Peripheral Neuropathy
Cobalamin is a cofactor for the enzyme Methylmalonyl-CoA mutase which converts methylmalonyl-CoA to succinyl-CoA. Succinyl-CoA enters the Krebs cycles and goes into nerves to make myelin. If no Vitamin B12, methylmalonyl-CoA goes on to form abnormal fatty acids and causes subacute degeneration of the nerves. Only B12 can correct this problem.
Therapeutic Uses of B12
Daily Requirements - 0.6-1.0mh/day; T1/2 ~ 1 year
Pernicious Anemia
Impaired GI absorption of B12
Gastrectomy
Corrosive Injury of GI mucosa
Fish tape worm: worm siphons off B12
Placebo abuse with B12, especially in elderly patients.
Folic Acid
Source in food yeast, egg yolk, liver and leafy vegetables
Folic Acid (F.A.) is absorbed in the small intestines.
F.A. is converted to tetrahydrofolate by dihydrofolate reductase.
Folic Acid deficiency (F.A. Deficiency) is also called Wills Disease. Deficiency may produce megaloblastic anemia; neural tube defect in fetus.
Therapeutic Uses of Folic Acid
1. Megaloblastic Anemia due to inadequate dietary intake of folic acid
Can be due to chronic alcoholism, pregnancy, infancy, impaired utilization: uremia, cancer or hepatic disease.
2. To alleviate anemia that is associated with dihydrofolate reductase inhibitors.
i.e. Methotrexate (Cancer chemotherapy), Pyrimethamine (Antimalarial) Administration of citrovorum factor (methylated folic acid) alleviates the anemia.
Therapeutic Uses of Folic Acid (cont)
3. Ingestion of drugs that interfere with intestinal absorption and storage of folic acid.
Mechanism- inhibition of the conjugases that break off folic acid from its food chelators. Ex. phenytoin, progestin/estrogens (oral contraceptives)
4. Malabsorption Sprue, Celiac disease, partial gastrectomy. 5. Rheumatoid arthritis increased folic acid demand or utilization.
You might also like
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- 9 Telephony Calls Over Bluetooth PDFDocument5 pages9 Telephony Calls Over Bluetooth PDFAdrul FauzanNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Kuliah 5 - Medicinal Herbal TherapyDocument18 pagesKuliah 5 - Medicinal Herbal TherapyAdrul FauzanNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Case 5Document1 pageCase 5Adrul FauzanNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Efectos de La Exposición in UteroDocument78 pagesEfectos de La Exposición in Uteroapi-3706745No ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The AutonomicDocument1 pageThe AutonomicAdrul FauzanNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- In 1992Document1 pageIn 1992Adrul FauzanNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Pharmaco KineticsDocument14 pagesPharmaco KineticsAdrul FauzanNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- InfluenzaDocument43 pagesInfluenzaAdrul FauzanNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- How To Control Our High Cholesterol and What Drug Can We DrinkDocument2 pagesHow To Control Our High Cholesterol and What Drug Can We DrinkAdrul FauzanNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Higher Algebra - Hall & KnightDocument593 pagesHigher Algebra - Hall & KnightRam Gollamudi100% (2)
- Higher Algebra - Hall & KnightDocument593 pagesHigher Algebra - Hall & KnightRam Gollamudi100% (2)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Clinical History TakingDocument36 pagesClinical History Takingapi-1964133775% (4)
- Faropenem - A New Oral Antibacterial Agent: Inpharma 1289 - 26 May 2001Document2 pagesFaropenem - A New Oral Antibacterial Agent: Inpharma 1289 - 26 May 2001Amàr AqmarNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Qingling Haung Resume - HcaDocument4 pagesQingling Haung Resume - Hcaapi-317607382No ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- المسار غير الجراحيDocument21 pagesالمسار غير الجراحيAhmad L YasinNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Daftar Obat & Alkes Trolley EmergencyDocument14 pagesDaftar Obat & Alkes Trolley EmergencyKomang Astrie100% (3)
- Medical-Surgical Nursing Assessment and Management of Clinical Problems 9e Chapter 40Document9 pagesMedical-Surgical Nursing Assessment and Management of Clinical Problems 9e Chapter 40sarasjunkNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Sle 5. 2014Document23 pagesSle 5. 2014Fahad Nauman SafirNo ratings yet
- Nle Tips MS PDFDocument11 pagesNle Tips MS PDFjthsNo ratings yet
- Left Thigh Pain: I. Chief Complaint/ Other ComplaintsDocument9 pagesLeft Thigh Pain: I. Chief Complaint/ Other ComplaintsDominic BristolNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Transgender SpeechDocument61 pagesTransgender Speechjreljosh1994100% (1)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Module 8 PERDEVDocument4 pagesModule 8 PERDEVmich100% (3)
- Ur MomDocument2 pagesUr MomZahranisa Zulfa SafiraNo ratings yet
- Management of Patients With Cerebrovascular Disorders: Ariel M. Ortuoste, RN, ManDocument115 pagesManagement of Patients With Cerebrovascular Disorders: Ariel M. Ortuoste, RN, ManarielleortuosteNo ratings yet
- Produk Parma 2Document2 pagesProduk Parma 2Anna ApriyantiNo ratings yet
- Scalp Acupuncture - MS1 To MS14 - Ok - Ok - OkDocument51 pagesScalp Acupuncture - MS1 To MS14 - Ok - Ok - Okmd_corona62100% (16)
- Biblical Counseling - Module 1Document26 pagesBiblical Counseling - Module 1lszalontaiNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Frank Ros The Lost Secrets of Ayurvedic AcupunctureDocument228 pagesFrank Ros The Lost Secrets of Ayurvedic AcupuncturePrasana Siva100% (6)
- Contoh Soal TOEFL PT AskrindoDocument2 pagesContoh Soal TOEFL PT AskrindoDeni HarmokoNo ratings yet
- PB - Using A Spirometer To Investigate Human Lung Function SsDocument6 pagesPB - Using A Spirometer To Investigate Human Lung Function SsMariam AymanNo ratings yet
- CBT DetailedDocument64 pagesCBT DetailedNazish KhanNo ratings yet
- NBDE Questions2Document98 pagesNBDE Questions2Bonnie SengNo ratings yet
- Anxiety Practice Qs - Sample AnswerDocument5 pagesAnxiety Practice Qs - Sample AnswerShanzay FatimaNo ratings yet
- AS R A B A - W T C M C: Hort Eview of Cupuncture and Ronchial Sthma Estern AND Raditional Hinese Edicine OnceptsDocument11 pagesAS R A B A - W T C M C: Hort Eview of Cupuncture and Ronchial Sthma Estern AND Raditional Hinese Edicine Onceptsaprilia ciet cietNo ratings yet
- DiferenteDocument5 pagesDiferenteGabriela ScarlatNo ratings yet
- Mark Pearson, Helen Wilson SandplayDocument150 pagesMark Pearson, Helen Wilson Sandplayankadi92% (13)
- Vethathiri - Holistic HealthDocument19 pagesVethathiri - Holistic HealthProf. Madhavan100% (2)
- Ethical Issues in Pediatric AnesDocument16 pagesEthical Issues in Pediatric AnesrejNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- 02 Feb 2019 RS Salewangang Maros (Orthopedi)Document13 pages02 Feb 2019 RS Salewangang Maros (Orthopedi)Adjhy Aji AchmadNo ratings yet
- Arum Hastuti Dwi Sofiati Jurnal Persalinan B.inggrisDocument5 pagesArum Hastuti Dwi Sofiati Jurnal Persalinan B.inggrisArum HastutiNo ratings yet
- John Nash LifeDocument8 pagesJohn Nash Lifek_a1990No ratings yet