Professional Documents
Culture Documents
Steel Manufacturing
Uploaded by
NDTInstructor100%(3)100% found this document useful (3 votes)
362 views29 pagessteel
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentsteel
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
100%(3)100% found this document useful (3 votes)
362 views29 pagesSteel Manufacturing
Uploaded by
NDTInstructorsteel
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 29
LOGO
Manufacturing Engineering and
Materials
Mechanical Behavior, Testing and
Manufacturing Properties of Materials.
Metal Alloys: Structure and Strengthening by
Heat Treatment
Ferrous Metals and Alloys: Production, General
Properties, and Applications
1
Materials Classification
Engineering
Materials
Metals
Ferrous
Steels
Stainless Steels
Nonferrous
Aluminum
Copper
Plastics
Thermoplastics
Thermosets
Elastomers
Ceramics and
others
Glass ceramics
Carbides
Diamond
Composites
Reinforced
plastics
Ceramic-matrix
Laminates
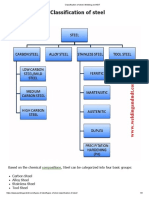
Ferrous
Materials
Carbon Steel
Low C.S
(C < 0.3 %)
Medium C.S (0.3 <
C <0.7)%
High C.S
(C > 0.7 %)
Alloy steel
Low A.S
(Alloying elements
up to 3 %)
High A.S
(Alloying elements
up to 7 %)
High Speed Steel
(HSS)
Cast Iron
Gray Cast Iron
White Cast Iron
Nodular Cast Iron
Malleable Cast Iron
Stainless Steel
(> 12 % Cr)
Austenitic S.S
Ferritic S.S
Martensitic S.S
Participation
Hardening S.S
Duplex S.S
Materials Classification
Behavior and manufacturing
properties of materials
Structure of
materials
Atomic bonds:
metallic, covaient,
and ionic
Crystalline
Amorphous
Mechanical
properties
Strength
Ductility
Hardness
Physical and chemical
properties
Density
Melting point
Thermal
conductivity
Property
modification
Heat treatment
Alloying
Surface treatment
Materials Classification
Mechanical Properties of Materials
Property Definition
Strength The ability of a material to bear an applied load.
Ductility The ability of a metal to deform without breaking.
Brittleness Tendency of a material to break without significant
deformation.
Hardness Ability to resist indentation.
Toughness The ability to absorb energy
Ductile to Brittle
transition
temperature
The temperature at which a metal fracture mode changes from
ductile to brittle.
Fatigue Strength The strength of a metal when exposed to repeated reversals of
cyclic stresses.
Soundness Freedom from discontinuities
Mechanical Properties of Materials
Typical Stress/Strain Curve - Steel
Steel Making
Row Materials for Production
Iron Ore
Limestone
Coke
Steel Making
The three raw materials are
dumped into a blast furnace.
Hot air (2000 F) is blasted into
the furnace, which helps drive
the chemical reaction. The
coke forms CO and the CO
reduces the iron oxide to iron.
The slag floats to the top and
the metal is transferred to
molds and cools. IT IS NOW
PIG IRON, ready for more iron
work or steelmaking.
Steel Making
To make steel you are simply removing more impurities, such as,
manganese, silicon, carbon, from the pig iron.
Impurities are removed by re-melting the metal and adding carbon,
steel scrap, and more limestone.
The metal can be melted using one of three methods
Open-Hearth furnace
Electric furnace
Basic Oxygen furnace. (BOF)
Open-Hearth Furnace
Uses a fuel to generate heat, and melt the metal.
Electric Furnace
Uses electric arc from
electrode to metal to heat
and melt it.
Can produce 60-90 tons of
steel per day.
Steel is higher quality than
open-hearth and BOF
Basic-Oxygen Furnace
Fastest steelmaking process can make
250 tons of steel / hour
Melted pig iron and scrap are poured
(charged) into a vessel.
Fluxing agents are added, like limestone.
The molten metal is blasted with pure
oxygen. This produces iron oxide which
then reacts with carbon to produce CO
and CO2. The slag floats to the top of the
metal.
Higher steel quality than open hearth.
Used to make plate, sheet, I-beam,
tubing and channel.
Steel Making
Steel Making
Casting of Ingots
While steel is still molten, it is poured into a mold.
The mold may be a square, rectangle or round. The
metal becomes an ingot in the mold.
The ingot will be removed from the mold and heated
uniformly to be rolled or formed into a final product.
While the molten metal cools, or solidifies, gasses
evolve and can affect the quality of the steel. This
leads to three types of steel: Killed Steel, Semi-Killed
Steel, and Rimmed Steel.
Casting of Ingots
This is a fully de-oxidized steel, and
thus, has no porosity.
Killed Steel
It is only partially de-oxidized, and
therefore, is a little more porous than
killed steel.
Semi Killed
Steel
The unwanted gasses form blowholes
around the rim Result in little or no
piping
Rimmed
Steel
Continuous Casting
Molten metal skips ingot
step, and goes directly from
the furnace to a tundish.
Metal solidifies in the mold.
The solidified metal then
goes through pinch rollers
that determine the final
form.
Continuous Casting
Benefits of Continuous Casting
Costs less to produce final product
Metal has more uniform composition and properties
than ingot processing.
Carbon and Alloy Steels
Carbon and alloying steels are the most commonly
used metals
The structural makeup and controlled processing of
these steels make them suitable for many different
functions.
Basic product shapes include plate, sheet, bar, wire,
tube, castings, and forgings.
Increasing the percentages of these elements in
steels, increases the properties they impart.
Carbon and Alloy Steels
Different elements are added to steels to given the
steel different properties.
The elements pass on properties such as harden-
ability, strength, hardness, toughness, wear
resistance, etc.
Some properties are beneficial while others are
detrimental.
Carbon and Alloy Steels
Effects of various elements in steels
Element Effect
Boron Improves hardenability without the loss of (or even with some
improvement in) machinability and formability.
Calcium Deoxidizes steels, improves toughness, and may improve formability
and machinability.
Carbon improves hardenability, strength, hardness, and wear resistance; it
reduces ductility, weldability, and toughness.
Cerium controls the shape of inclusions and improves toughness in high-
strength low alloy steels; it deoxidizes steels.
Chromium improves toughness, hardenability, wear and corrosion resistance,
and high-temperature strength; it increases the depth of the
hardness penetration resulting from heat treatment by promoting
carburization.
Carbon and Alloy Steels
Effects of various elements in steels
Element Effect
Cobalt improves strength and hardness at elevated temperatures.
Copper improves resistance to atmospheric corrosion and, to a lesser
extent, increases strength with little loss in ductility; it adversely
affects the hot-working characteristics and surface quality.
Lead improves machinability; it causes liquid-metal embrittlement.
Magnesium has the same effects as cerium.
Manganese improves hardenability, strength, abrasion resistance, and
machinability; it deoxidizes the molten steel, reduce shot shortness,
and decreases weldability.
Carbon and Alloy Steels
Effects of various elements in steels
Element Effect
Molybdenum improves hardenability, wear resistance, toughness, elevated-
temperature strength, creep resistance, and hardness; it minimizes
temper embrittlement.
Nickel improves strength, toughness, and corrosion resistance; it improves
hardenability.
Niobium
(columbium)
imparts fineness of grain size and improves strength and impact
toughness; it lowers transition temperature and may decrease
hardenability.
Phosphorus improves strength, hardenability, corrosion resistance, and
machinability; it severely reduces ductility and toughness.
Selenium improves machinability.
Carbon and Alloy Steels
Effects of various elements in steels
Element Effect
Silicon improves strength, hardness, corrosion resistance, and electrical
conductivity; it decreases magnetic-hysteresis loss, machinability,
and cold formability.
Sulfur Improves machinability when combined with manganese; it lowers
impact strength and ductility and impairs surface quality and
weldability.
Titanium improves hardenability; it deoxidizes steels.
Vanadium improves strength, toughness, abrasion resistance, and hardness at
elevated temperatures; it inhibits grain growth during heat
treatment.
Tungsten has the same effects as cobalt.
Carbon and Alloy Steels
Carbon steels
Carbon steels are group by their percentage of
carbon content per weight. The higher the carbon
content the greater the hardness, strength and wear
resistance after heat treatment.
Carbon steels are classified to:
Low Carbon Steel
Medium Carbon Steel
High Carbon Steel
Carbon and Alloy Steels
Low Carbon Steel
Also called mild
steels, has less
than 0.30%
carbon. Used in
everyday
industrial
products like
bolts, nuts, sheet,
plate and tubes.
Medium Carbon
Steel
has 0.30% to
0.60% carbon.
Used for jobs
requiring higher
strength such as
machinery,
automotive
equipment parts,
and metalworking
equipment.
High Carbon Steel
has more than
0.60% carbon.
Used parts that
require the
highest strength,
hardness, and
wear resistance.
Once
manufactured
they are heat
treated and
tempered
Carbon and Alloy Steels
27
Alloy Steels
are steels that contain significant amounts of
alloying elements.
Alloy steels are classified to:
High strength low alloy steels
Microalloyed steels
Nanoalloyed steels
Carbon and Alloy Steels
High-strength, low-
alloy steels (HSLA)
were developed
to improve the
ratio of strength
to weight.
Commonly used
in automobile
bodies and in the
transportation
industry (the
reduced weight
makes for better
fuel economy ).
Microalloyed steels
Provide superior
properties
without the use of
heat treating.
When cooled
carefully these
steels develop
enhanced and
consistent
strength.
Nanoalloyed steels
have extremely
small grain size
(10-100 nm).
Since their
synthesis is done
at an atomic level
their properties
can be controlled
specifically.
Stainless Steels
29
You might also like
- Classifying Common Ferrous Metals and AlloysDocument26 pagesClassifying Common Ferrous Metals and AlloysLira AgbonNo ratings yet
- Production of Steel and Its ClassificationDocument36 pagesProduction of Steel and Its Classificationabdullah anwar100% (1)
- Carbon and Alloy Steel PDFDocument52 pagesCarbon and Alloy Steel PDFmaz234100% (3)
- Classification of Steel - Welding and NDTDocument3 pagesClassification of Steel - Welding and NDTAshif Iqubal100% (1)
- Basic Metallurgy ExplainedDocument32 pagesBasic Metallurgy Explainedvarundevil87No ratings yet
- Engg Metallurgy Lecture 5Document54 pagesEngg Metallurgy Lecture 5Patil Sudheer GowdNo ratings yet
- Cast IronDocument21 pagesCast Irondellibabu509No ratings yet
- Metallurgy For Non Metallurgist 1Document68 pagesMetallurgy For Non Metallurgist 1aravind_mett100% (7)
- Steel and Iron Production ProcessesDocument59 pagesSteel and Iron Production ProcessesGianardo Satria PrimandanuNo ratings yet
- End Splitting During Long Products Rolling - Billet Quality of Rolling ProcessDocument27 pagesEnd Splitting During Long Products Rolling - Billet Quality of Rolling ProcessJorge MadiasNo ratings yet
- Steel Manufacturing ProcessDocument28 pagesSteel Manufacturing ProcesstranngNo ratings yet
- Iron and Steel Manufacturing ProcessDocument28 pagesIron and Steel Manufacturing ProcessMarnel Roy Mayor78% (32)
- Steel MakingDocument35 pagesSteel MakingBharichalo007No ratings yet
- Task2.2 Melting EfficiencyDocument47 pagesTask2.2 Melting EfficiencyemregnesNo ratings yet
- Unit 1 Heat Treatment of SteelsDocument207 pagesUnit 1 Heat Treatment of SteelsAishwarya JanbandhuNo ratings yet
- 0 Introduction To MetalDocument139 pages0 Introduction To MetalMichael TanjayaNo ratings yet
- Cryogenic Steel Properties and ApplicationsDocument0 pagesCryogenic Steel Properties and ApplicationsidienNo ratings yet
- Macrostructure Defect in Continuous Steel CastingDocument13 pagesMacrostructure Defect in Continuous Steel CastingHasna RiazNo ratings yet
- Roll Breakage DetectionDocument7 pagesRoll Breakage Detectionzubair ahmadNo ratings yet
- Steel Making Processes Post-Solidification Treatment: - ESR (Electro-Slag Refining) - VAR (Vacuum-Arc Remelting)Document27 pagesSteel Making Processes Post-Solidification Treatment: - ESR (Electro-Slag Refining) - VAR (Vacuum-Arc Remelting)Asher Ahmed100% (1)
- Forging and Sheet Metal FormingDocument83 pagesForging and Sheet Metal FormingnvemanNo ratings yet
- Ferrous Alloy Types and PropertiesDocument72 pagesFerrous Alloy Types and PropertiesSneha Kriti100% (3)
- Steel Making - Nptel PDFDocument214 pagesSteel Making - Nptel PDFanurag3069100% (3)
- Heat TreatmentDocument30 pagesHeat Treatmentjhamlal100% (2)
- Lecture Notes Iron Making (PCMT4303) - 6th Sem BTech (Metallurgy)Document158 pagesLecture Notes Iron Making (PCMT4303) - 6th Sem BTech (Metallurgy)mandakini baskey100% (2)
- Heat TreatmentDocument44 pagesHeat Treatmentmurari100% (2)
- MTech Industrial Metallurgy E Learning Executive Summary &FAQsDocument6 pagesMTech Industrial Metallurgy E Learning Executive Summary &FAQsakshukNo ratings yet
- Heat Treatment of Steel TTT CurveDocument59 pagesHeat Treatment of Steel TTT CurveINSTECH Consulting100% (1)
- Billet DefectsDocument29 pagesBillet Defectsnqvinh_dnNo ratings yet
- On Steel MakingDocument58 pagesOn Steel Makingallan arthur bare100% (1)
- METALLURGY AND MATERIALS PROPERTIESDocument55 pagesMETALLURGY AND MATERIALS PROPERTIESTina Miller100% (2)
- Rolling DefectsDocument8 pagesRolling Defectsvelavansu100% (2)
- DefectsDocument51 pagesDefectsCollege BoysNo ratings yet
- Precipitation HardeningDocument6 pagesPrecipitation Hardeningjyoti swaroop repaka100% (9)
- Introduction To Steel MakingDocument44 pagesIntroduction To Steel MakingDrTrinath TalapaneniNo ratings yet
- MetallurgyDocument190 pagesMetallurgyJose J. Nuñez100% (2)
- Welding MetallurgyDocument69 pagesWelding MetallurgyManish Sharma100% (8)
- Roll Pass Design Evauluation Using Software ApplicationDocument34 pagesRoll Pass Design Evauluation Using Software ApplicationAbed Alrahman NashwanNo ratings yet
- Casting Defect in Slab PDFDocument55 pagesCasting Defect in Slab PDFBhoomaiah SunkenapalliNo ratings yet
- Rolling Mill DrivesDocument14 pagesRolling Mill DrivesMoinul Haque Riyad0% (1)
- Physical MetallurgyDocument670 pagesPhysical MetallurgyprasadNo ratings yet
- Rolling of Metals Process and Principles (With Diagram)Document11 pagesRolling of Metals Process and Principles (With Diagram)SUDIPTA BHATTACHARJEENo ratings yet
- 10-Low Alloy Steel PDFDocument32 pages10-Low Alloy Steel PDFIdes Trian100% (1)
- Tool SteelsDocument35 pagesTool SteelsHandrizaHanifAsyrafiNo ratings yet
- A New Method For Roll Pass Design Optimi PDFDocument12 pagesA New Method For Roll Pass Design Optimi PDFFarooq Ameer Jordan WalaNo ratings yet
- Engineering Materials: Learning ObjectivesDocument11 pagesEngineering Materials: Learning Objectives38Zeeshan ZameerNo ratings yet
- Basic MetallurgyDocument72 pagesBasic MetallurgyMuhammad Haris100% (1)
- Copper and Tin in Steel Scrap RecyclingDocument15 pagesCopper and Tin in Steel Scrap RecyclingakshukNo ratings yet
- Killed and Capped Steel IngotsDocument5 pagesKilled and Capped Steel IngotsalacalleNo ratings yet
- Material Science and EngineeringDocument1 pageMaterial Science and EngineeringRap itttt88% (8)
- Effect of Alloying Elements On Steel Properties (SubsTech)Document2 pagesEffect of Alloying Elements On Steel Properties (SubsTech)hguptabhel100% (1)
- Iron-carbon phase diagram guideDocument9 pagesIron-carbon phase diagram guideNagamuthu PandianNo ratings yet
- Tramp Elements and Billet CarckingDocument7 pagesTramp Elements and Billet CarckingOmar TahaNo ratings yet
- Modern Steel - Lecture 1Document54 pagesModern Steel - Lecture 1Jojo Hany100% (1)
- CH 5 - Ferrous Metals and AlloysDocument45 pagesCH 5 - Ferrous Metals and AlloysYhan SombilonNo ratings yet
- 211 2aDocument33 pages211 2aMada ChohNo ratings yet
- Carbon steelDocument9 pagesCarbon steelArfanAliNo ratings yet
- FC-06-Engineering Material & Metallurgy PDFDocument431 pagesFC-06-Engineering Material & Metallurgy PDFsomnath ghosh100% (1)
- PAUT Vs RadiographyDocument1 pagePAUT Vs RadiographyNDTInstructorNo ratings yet
- TunisiaDocument4 pagesTunisiaNDTInstructorNo ratings yet
- Forging DefectsDocument1 pageForging DefectsNDTInstructorNo ratings yet
- Job Description NDTDocument4 pagesJob Description NDTNDTInstructorNo ratings yet
- Color Vision TestDocument1 pageColor Vision TestNDTInstructorNo ratings yet
- Color Vision TestDocument1 pageColor Vision TestNDTInstructorNo ratings yet
- NigieriaDocument12 pagesNigieriaNDTInstructorNo ratings yet
- Piping 4Document1 pagePiping 4NDTInstructorNo ratings yet
- Api 580Document1 pageApi 580NDTInstructorNo ratings yet
- Piping 3Document1 pagePiping 3NDTInstructorNo ratings yet
- H300 Introduction (Si Units) : Para. 304.3.3Document2 pagesH300 Introduction (Si Units) : Para. 304.3.3NDTInstructorNo ratings yet
- Measuring Residual Magnetic Fields with Field Indicators and Gauss MetersDocument1 pageMeasuring Residual Magnetic Fields with Field Indicators and Gauss MetersNDTInstructorNo ratings yet
- (A) Elastic Behavior. The Assumption That DisplaceDocument1 page(A) Elastic Behavior. The Assumption That DisplaceNDTInstructorNo ratings yet
- Piping 4Document1 pagePiping 4NDTInstructorNo ratings yet
- A309 Bolting: Appendix F Para. F309 Paragraph 309.1 Para. 309.1 Para. A335.2Document1 pageA309 Bolting: Appendix F Para. F309 Paragraph 309.1 Para. 309.1 Para. A335.2NDTInstructorNo ratings yet
- Eddify Lyft Presentation DocumentDocument4 pagesEddify Lyft Presentation DocumentBernardo FariasNo ratings yet
- 344.2 Visual Examination: Para. 344.7Document1 page344.2 Visual Examination: Para. 344.7NDTInstructorNo ratings yet
- Shell Settlement EvaluationDocument1 pageShell Settlement EvaluationNDTInstructorNo ratings yet
- Solutionstainlesssteelweldinspection 170124181624Document13 pagesSolutionstainlesssteelweldinspection 170124181624NDTInstructorNo ratings yet
- Tank Inspection SolutionDocument7 pagesTank Inspection SolutionNDTInstructorNo ratings yet
- General TankDocument2 pagesGeneral TankNDTInstructorNo ratings yet
- API Individual Certification Programs: Mohamed Karim RamyDocument1 pageAPI Individual Certification Programs: Mohamed Karim RamyNDTInstructorNo ratings yet
- Procedure CFVDocument13 pagesProcedure CFVNDTInstructorNo ratings yet
- Optimized Performance For Wall Thickness and LiftoffDocument1 pageOptimized Performance For Wall Thickness and LiftoffNDTInstructorNo ratings yet
- Non-Destructive Testing: Sample Questions For Conduct of Examinations at Levels 1 and 2Document242 pagesNon-Destructive Testing: Sample Questions For Conduct of Examinations at Levels 1 and 2darqm589% (18)
- Olympus Multiscan Ms 5800 Er1uDocument2 pagesOlympus Multiscan Ms 5800 Er1uNDTInstructorNo ratings yet
- Inspections and Audits For Boilers and Pressure VesselsDocument2 pagesInspections and Audits For Boilers and Pressure VesselsNDTInstructorNo ratings yet
- API Individual Certification Programs: Mohamed Karim RamyDocument1 pageAPI Individual Certification Programs: Mohamed Karim RamyNDTInstructorNo ratings yet
- Job Hazard AnalysisDocument5 pagesJob Hazard AnalysisNDTInstructorNo ratings yet
- Vision CertificateDocument1 pageVision CertificateNDTInstructorNo ratings yet
- Callister Ch09Document90 pagesCallister Ch09Nemish KanwarNo ratings yet
- Manufacturing Routes For Metallic Foams: John BanhartDocument6 pagesManufacturing Routes For Metallic Foams: John BanhartJuan Jose Martinez PadillaNo ratings yet
- Methods For Waste Waters Treatment in Textile IndustryDocument5 pagesMethods For Waste Waters Treatment in Textile Industryjpsingh75No ratings yet
- Flow Sensor Technical Guide BookDocument20 pagesFlow Sensor Technical Guide Books12originalNo ratings yet
- AkzoNobel - Colloidal Silica For Adhesives BrochureDocument6 pagesAkzoNobel - Colloidal Silica For Adhesives BrochureCarlos GuerreroNo ratings yet
- Aes Secondary Emulsifier: Safety Data SheetDocument8 pagesAes Secondary Emulsifier: Safety Data Sheetgerardo sifuentesNo ratings yet
- Nuclear Medicine Inc.'s Iodine Value Chain AnalysisDocument6 pagesNuclear Medicine Inc.'s Iodine Value Chain AnalysisPrashant NagpureNo ratings yet
- CE2155 - 01 Mechanic of Materials (Part 3)Document18 pagesCE2155 - 01 Mechanic of Materials (Part 3)Julia100% (1)
- SSIP GR 11 Acids N BasesDocument10 pagesSSIP GR 11 Acids N BasesMangwane Sello100% (1)
- PhysioEx Exercise 1 Activity 4Document3 pagesPhysioEx Exercise 1 Activity 4Дмитро МарчукNo ratings yet
- IJREI_Thermal modelling of three stage vapour compression cascade refrigeration system using entropy generation principle for reducing global warming and ozone depletion using ecofriendly refrigerants for semen preservationDocument7 pagesIJREI_Thermal modelling of three stage vapour compression cascade refrigeration system using entropy generation principle for reducing global warming and ozone depletion using ecofriendly refrigerants for semen preservationIjrei JournalNo ratings yet
- Ele541 Kje412 SJ 12Document13 pagesEle541 Kje412 SJ 12Mu'izz KaharNo ratings yet
- MasterSeal P 698 MSDS - EN-23.11.2022-Rev1.2Document11 pagesMasterSeal P 698 MSDS - EN-23.11.2022-Rev1.2recep kablanNo ratings yet
- Formula Sheet HTDocument8 pagesFormula Sheet HTChristopher FernandesNo ratings yet
- Refineria de Cartagena (Reficar) Refinery Expansion - Hydrocarbons TechnologyDocument3 pagesRefineria de Cartagena (Reficar) Refinery Expansion - Hydrocarbons TechnologyGjorgeluisNo ratings yet
- Elisa: Enzyme-Linked Immunosorbent AssayDocument12 pagesElisa: Enzyme-Linked Immunosorbent AssayAmitNo ratings yet
- 04 - Spektroskopi UV-Vis - 3Document16 pages04 - Spektroskopi UV-Vis - 3muktadi-amri-8721No ratings yet
- Sircal Product BrochureDocument1 pageSircal Product Brochuresaleem malikNo ratings yet
- Safety Data Sheet: Product Name: MOBIL SHC RARUS 68Document10 pagesSafety Data Sheet: Product Name: MOBIL SHC RARUS 68Daniel Rodriguez GutierrezNo ratings yet
- Admixtures and Shotcrete DurabilityDocument7 pagesAdmixtures and Shotcrete DurabilityMulyawan WIdiasmanNo ratings yet
- Origins of Rheology: A Brief Look at the Evolution of the Study of Material FlowDocument9 pagesOrigins of Rheology: A Brief Look at the Evolution of the Study of Material FlowAmlan PalNo ratings yet
- Cutting Tools TypeDocument3 pagesCutting Tools TypeneurraNo ratings yet
- Biostimulation Treatments of Hydrocarbon-Contaminated SoilDocument6 pagesBiostimulation Treatments of Hydrocarbon-Contaminated SoilGeorgian-Răzvan CheșcaNo ratings yet
- Small STNTechnical ManualDocument8 pagesSmall STNTechnical ManualMajid KhanNo ratings yet
- Eutronic - Arc - Spray 4HFDocument4 pagesEutronic - Arc - Spray 4HFMuhammad irfanNo ratings yet
- Spectrofotometru SpectroDirect (De La Lovibond)Document360 pagesSpectrofotometru SpectroDirect (De La Lovibond)FlaviusNo ratings yet
- Accessing The Chemical Properties of Clay Deposits in Ekiti StateDocument41 pagesAccessing The Chemical Properties of Clay Deposits in Ekiti StateJayla CroninNo ratings yet
- SPE Papers Well DeliverabilityDocument279 pagesSPE Papers Well DeliverabilitySyed Ahmed FlareNo ratings yet
- Bio Process Problem 1Document1 pageBio Process Problem 1AshenafiNo ratings yet
- HS Analysis of AminesDocument10 pagesHS Analysis of AminesВиталий ШариповNo ratings yet